

(1)¼Ó2 mLĖ®¼°Š”»š¼ÓČȵÄŌŅņ___________________________________________________”£
(2)ČōŗöĀŌ¼Ó2 mIĖ®¼°Š”»š¼ÓČČ£¬½«»į²śÉśŗģ×ŲÉ«ÕōĘų£¬Š“³ö²śÉśŗģ×ŲÉ«ÕōĘųµÄ·½³ĢŹ½___________________________________________________________________”£
(3)ŌŚUŠĪ¹ÜÖŠŹÕ¼Æµ½µÄäåŅŅĶé³ŹŗģŗÖÉ«£¬ŌŅņŹĒ___________________________________”£
(4)¾»»ÆäåŅŅĶéµÄ·½·ØŹĒ__________________________________________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŅŃÖŖ£ŗCH3CH2OH+NaBr+H2SO4£ØÅØ£©
ŅŃÖŖ£ŗCH3CH2OH+NaBr+H2SO4£ØÅØ£©| ”÷ |
| ”÷ |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 äåŅŅĶéŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬Ęä·ŠµćĪŖ38.4”ę£®ÖʱøäåŅŅĶéµÄŅ»ÖÖ·½·ØŹĒŅŅ“¼ÓėĒāäåĖį·“Ó¦£¬øĆ·“Ó¦Źµ¼ŹĶس£ŹĒÓĆäå»ÆÄĘÓėŅ»¶ØÅØ¶ČµÄĮņĖįŗĶŅŅ“¼·“Ó¦£®Ä³æĪĶāŠ”×éÓūŌŚŹµŃéŹŅÖʱøäåŅŅĶéµÄ×°ÖĆČēĶ¼£¬ŹµŃé²Łāō²½ ÖčČēĻĀ£ŗ
äåŅŅĶéŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬Ęä·ŠµćĪŖ38.4”ę£®ÖʱøäåŅŅĶéµÄŅ»ÖÖ·½·ØŹĒŅŅ“¼ÓėĒāäåĖį·“Ó¦£¬øĆ·“Ó¦Źµ¼ŹĶس£ŹĒÓĆäå»ÆÄĘÓėŅ»¶ØÅØ¶ČµÄĮņĖįŗĶŅŅ“¼·“Ó¦£®Ä³æĪĶāŠ”×éÓūŌŚŹµŃéŹŅÖʱøäåŅŅĶéµÄ×°ÖĆČēĶ¼£¬ŹµŃé²Łāō²½ ÖčČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¹ć¶«Ź”Ö“ŠÅ֊ѧ2011£2012ѧğø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧĄķæĘŹŌĢā ĢāŠĶ£ŗ058
ŅŃÖŖ£ŗ¢ŁäåŅŅĶéµÄ·Šµć38£®4”ę
¢ŚNaBr£«H2SO4(ÅØ)![]() NaHSO4£«HBr”ü
NaHSO4£«HBr”ü
¢Ū2HBr£«H2SO4(ÅØ)![]() Br2£«SO2”ü£«2H2O
Br2£«SO2”ü£«2H2O
ŹµŃéŹŅÖʱøäåŅŅĶé(C2H5Br)µÄ×°ÖĆČēĶ¼£¬²½ÖčČēĻĀ£ŗ

¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£¬Ļņ×°ÖĆĶ¼ĖłŹ¾µÄUŠĪ¹ÜŗĶ“óÉÕ±ÖŠ¼ÓČė±łĖ®£»
¢ŚŌŚŌ²µ×ÉÕĘæÖŠ¼ÓČė10 mL””95£„ŅŅ“¼”¢28 mL””78£„ÅØĮņĖį£¬Č»ŗó¼ÓČėŃŠĻøµÄ13 gäå»ÆÄĘŗĶ¼øĮ£Ėé“Éʬ£»
¢ŪŠ”ŠÄ¼ÓČČ£¬Ź¹Ęä³ä·Ö·“Ó¦£®
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃéÓĆŅŅ“¼”¢ÅØĮņĖį”¢äå»ÆÄĘÖĘČ”äåŅŅĶéµÄ×ܵĻÆѧ·½³ĢŹ½ĪŖ£ŗ________£®
(2)·“Ó¦Ź±ČōĪĀ¶Č¹żøߣ¬æÉ擵½ÓŠŗģ×ŲÉ«ĘųĢå²śÉś£¬øĆĘųĢå»ÆѧŹ½ĪŖ£ŗ________£®
(3)ĪŖĮĖøüŗƵÄæŲÖĘĪĀ¶Č£¬³żÓĆĶ¼Ź¾µÄŠ”»š¼ÓČČĶā£¬øüŗĆµÄ¼ÓČČ·½Ź½ĪŖ________£®
(4)¢Ł·“Ó¦½įŹųŗó£¬UŠĪ¹ÜÄŚ“ÖÖʵÄC2H5Br³Ź×Ų»ĘÉ«£®ĪŖĮĖ³żČ„“Ö²śĘ·ÖŠµÄŌÓÖŹ£¬æÉŅŌŃ”ŌńĻĀĮŠŹŌ¼ĮÖŠµÄ________(Ģī×ÖÄø)£¬ĖłŠčµÄÖ÷ŅŖ²£Į§ŅĒĘ÷ĪŖ________£®
A£®NaOHČÜŅŗ
B£®Na2SO3ČÜŅŗ
C£®CCl4
¢ŚŅŖ½ųŅ»²½ÖĘµĆ“æ¾»µÄC2H5Br£¬æÉÓĆĖ®Ļ“£¬Č»ŗó________(Ģī²Ł×÷Ćū³Ę)£¬ŌŁ¼ÓČėĪŽĖ®CaCl2£¬×īŗó½ųŠŠ________(Ģī²Ł×÷Ćū³Ę)£®
(5)ĻĀĮŠ¼øĻīŹµŃé²½Öč£¬æÉÓĆÓŚ¼ģŃéäåŅŅĶéÖŠäåŌŖĖŲ£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£ŗȔɣĮæäåŅŅĶé£¬Č»ŗó________(ĢīŠņŗÅ)£®
¢Ł¼ÓČČ£»¢Ś¼ÓČėAgNO3ČÜŅŗ£»¢Ū¼ÓČėĻ”HNO3Ėį»Æ£»¢Ü¼ÓČėNaOHČÜŅŗ£»¢ŻĄäČ“
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”¶«±±Ź¦“óø½ÖŠ2009½ģøßČżµŚĖÄ“ĪĆžµ×æ¼ŹŌ(»Æѧ) ĢāŠĶ£ŗ058
ŹµŃéŹŅÖʱøäåŅŅĶé(C2H5Br)µÄŌĄķĪŖC2H5OH£«NaBr£«H2SO4![]() NaHSO4£«C2H5Br£«H2O£¬×°ÖĆČēĶ¼(ŅŃÖŖäåŅŅĶéµÄ·Šµć38.4”ę)ŗĶ²½ÖčČēĻĀ£ŗ
NaHSO4£«C2H5Br£«H2O£¬×°ÖĆČēĶ¼(ŅŃÖŖäåŅŅĶéµÄ·Šµć38.4”ę)ŗĶ²½ÖčČēĻĀ£ŗ
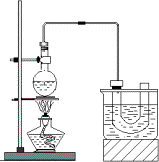
¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£¬Ļņ×°ÖĆĶ¼ĖłŹ¾µÄUŠĪ¹ÜŗĶ“óÉÕ±ÖŠ¼ÓČė±łĖ®£»¢ŚŌŚŌ²µ×ÉÕĘæÖŠ¼ÓČėŃŠĻøµÄ13 gäå»ÆÄĘŗĶ¼øĮ£Ėé“Éʬ£¬Č»ŗó¼ÓČė10 mL””95£„ŅŅ“¼”¢28 mL””78£„ÅØĮņĖį£»¢ŪŠ”ŠÄ¼ÓČČ£¬Ź¹Ęä³ä·Ö·“Ó¦£®
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)·“Ó¦Ź±ČōĪĀ¶Č¹żøߣ¬æÉ擵½ÓŠŗģ×ŲÉ«ĘųĢå²śÉś£¬øĆĘųĢå·Ö×ÓŹ½ĪŖ________
(2)ĪŖĮĖøüŗƵÄæŲÖĘ·“Ó¦ĪĀ¶Č£¬³żÓĆĶ¼Ź¾µÄŠ”»š¼ÓČČ£¬øüŗĆµÄ¼ÓČČ·½Ź½ŹĒ________£®
(3)UŠĶ¹ÜÄŚæɹŪ²ģµ½µÄĻÖĻóŹĒ________£®
(4)·“Ó¦½įŹųŗó£¬UŠĪ¹ÜÖŠ“ÖÖʵÄC2H5Br³Ź×Ų»ĘÉ«£®ĪŖĮĖ³żČ„“Ö²śĘ·ÖŠµÄŌÓÖŹ£¬æÉŃ”ŌńĻĀĮŠŹŌ¼ĮÖŠµÄ________(ĢīŠņŗÅ)£®
A£®NaOHČÜŅŗ””B£®H2O””C£®Na2SO3ČÜŅŗ””D£®CCl4
ĖłŠčµÄÖ÷ŅŖ²£Į§ŅĒĘ÷ŹĒ________(ĢīŅĒĘ÷Ćū³Ę)£®ŅŖ½ųŅ»²½ÖĘµĆ“æ¾»µÄC2H5Br£¬æÉÓĆĖ®Ļ“”¢·ÖŅŗ£¬Č»ŗóĻņÓĶ²ćÖŠ¼ÓČėĪŽĖ®CaCl2£¬ŌŁ½ųŠŠ________(Ģī²Ł×÷Ćū³Ę)£®
(5)ĻĀĮŠ¼øĻīŹµŃé²½Öč£¬æÉÓĆÓŚ¼ģŃéäåŅŅĶéÖŠäåŌŖĖŲ£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£ŗȔɣĮæäåŅŅĶé£¬Č»ŗó________(Ģī“śŗÅ)£®
¢Ł¼ÓČČ£»¢Ś¼ÓČėAgNO3ČÜŅŗ£»¢Ū¼ÓČėĻ”HNO3Ėį»Æ£»¢Ü¼ÓČėNaOHČÜŅŗ£»¢ŻĄäČ“
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com