
| �¶�/K | 298 | 398 | 498 | �� |
| ƽ�ⳣ����K�� | 4.1��105 | K1 | K2 | �� |
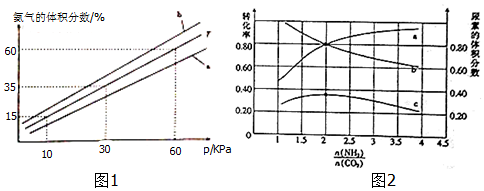
���� ��1����֪����2NO��g��+3H2��g��?2NH3��g��+O2��g����H1=-272.9kJ•mol-1����2H2��g��+O2��g���T2H2O��g����H2=-483.6kJ•mol-1���ڡ�3-�١�2�õ�4NH3��g��+5O2��g���T4NO��g��+6H2O��g�������ݸ�˹���������㼴�ɣ�
��2��ƽ�ⳣ�����¶ȱ仯����ѹǿ��Ũ�ȱ仯�أ�ƽ�ⳣ���仯��Ϸ�Ӧһ��ԭ�������жϣ�
��3��A����ͼ��֪���¶�һ��ʱ��ѹǿ�������������������
B���ϳɰ��Ƿ��ȷ�Ӧ��ѹǿһ��ʱ�������¶�ƽ��������Ӧ�����ƶ���ƽ��ʱ�����������������
C.500���¶�ʱ��Ӧ���ʼӿ켰����������ã�����ӦΪ���ȷ�Ӧ���¶�Խ�ͣ�������ת����Խ�ߣ�
D����ͬ���ʱ�ʾ����������֮�ȵ��ڻ�ѧ������֮�ȣ�˵�����淴Ӧ����ƽ�⣻
��μӷ�Ӧ�ĵ���Ϊn mol����
N2��g��+3H2��g��?2NH3��g��
��ʼ��mol����10 40 0
ת����mol����n 3n 2n
ƽ�⣨mol����10-n 40-3n 2n
����ƽ��ʱ�����������������ʽ����n���������㵪����ת���ʣ�
��4����4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��4NO+3O2+2H2O=4HNO3���ɵã�NH3+2O2=HNO3+H2O�����ݷ��̼��㣻
��5����ͼ��֪��������Ϊn��NH3����n��CO2��=2ʱ�����ص���������������ת����Ϊ80%���Դ˼���������·�Ӧ��ƽ�ⳣ��K��
��� �⣺��1����֪����2NO��g��+3H2��g��?2NH3��g��+O2��g����H1=-272.9kJ•mol-1����2H2��g��+O2��g���T2H2O��g����H2=-483.6kJ•mol-1���ڡ�3-�١�2�õ�4NH3��g��+5O2��g���T4NO��g��+6H2O��g�������ݸ�˹���ɡ�H3=3����-483.6kJ•mol-1��-2����-272.9kJ•mol-1��=-905.0KJ•mol-1���ʴ�Ϊ��-905.0KJ•mol-1��
��2����һ��������ܱ������У��������»�ѧ��Ӧ��N2��g��+3H2��g��?2NH3��g����H��0����Ӧ�Ƿ��ȷ�Ӧ������ͼ�����ݷ������¶����ߣ�ƽ��������У�ƽ�ⳣ����С��K1��K2���ʴ�Ϊ�������÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���ƽ�ⳣ����С��
��3����A����ͼ��֪���¶�һ��ʱ��ѹǿ�������������������A��ȷ��
B��ͼB��T=500�棬�ϳɰ��Ƿ��ȷ�Ӧ��ѹǿһ��ʱ�������¶�ƽ��������Ӧ�����ƶ���ƽ��ʱ��������������������¶�Ϊ450��ʱ��Ӧ��������b����B��ȷ��
C.500���¶�ʱ��Ӧ���ʼӿ켰����������ã�����ӦΪ���ȷ�Ӧ���¶�Խ�͵�����ת����Խ�ߣ����²����ڵ�����ת������C����
D���� 2v����H2��=3v����NH3������ͬ���ʱ�ʾ����������֮�ȵ��ڻ�ѧ������֮�ȣ�˵�����淴Ӧ����ƽ�⣬3v����H2��=2v����NH3��ʱ����˵�����淴Ӧ����ƽ�⣬��D����
��ѡ��AB��
����μӷ�Ӧ�ĵ���Ϊn mol����
N2��g��+3H2��g��?2NH3��g��
��ʼ��mol����10 40 0
ת����mol����n 3n 2n
ƽ�⣨mol����10-n 40-3n 2n
��$\frac{2n}{10-n+40-3n+2n}$��100%=25%�����n=5�����Ե�����ת����Ϊ$\frac{5}{10}$��100%=50%��
�ʴ�Ϊ��AB��50%��
��4����4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��4NO+3O2+2H2O=4HNO3�ɵã�NH3+2O2=HNO3+H2O���������ϵ�ԭ�ϱȽ�ԭ�Ϸ���һ�����з�Ӧ�������ܱ������н��У��������ʲ�����罻����������1molHNO3��molH2O�����������������Ϊ��$\frac{63}{63+18}$��100%=77.8%��
�ʴ�Ϊ��77.8%��
��5����ͼ��֪��������Ϊn��NH3����n��CO2��=2ʱ�����ص������������Ұ�����ת����Ϊ80%��
2NH3��g��+CO2��g��=CO��NH2��2��g��+H2O��g��
��ʼŨ�ȣ�mol��L-1 �� 1 0.5 0 0
�仯Ũ�ȣ�ol��L-1 �� 0.8 0.4 0.4 0.4
ƽ��Ũ�ȣ�mol��L-1 �� 0.2 0.1 0.4 0.4
�������·�Ӧ��ƽ�ⳣ��K=$\frac{0.4��0.4}{0.1��0��{2}^{2}}$=40��
�ʴ�Ϊ��2��40��
���� ���⿼�����Ȼ�ѧ����ʽ��˹���ɵļ���Ӧ�ã���ѧ��Ӧ�����仯ͼ�������ƽ���־��ƽ�ⳣ����ƽ�������жϼ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
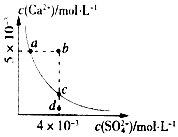
| A�� | ���κ���Һ�У�����CaSO4������������c��Ca2+����c��SO42-��һ����� | |
| B�� | d����Һͨ���������Ա䵽c�� | |
| C�� | a���Ӧ��Ksp����c���Ӧ��Ksp | |
| D�� | b�㽫�г������ɣ�ƽ�����Һ��c��SO42-��һ������3��10-3mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��25�������ˮ�У�FeS���ܽ����� | |
| B�� | 25��ʱ��CuS�ı�����Һ��Cu2+��Ũ��ԼΪ1.14��10-18 mol•L-1 | |
| C�� | ����FeS��������������ȥ��ˮ�е�Cu2+ | |
| D�� | �����ʵ���Ũ����ͬ��FeCl2��ZnCl2���Һ�м�������Na2S��ֻ��FeS�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HC1O | H2CO3 |
| KSp ��Ka | Ksp=1.8��10-10 | Ksp=2.0��10-12 | Ka=1.8��10 -5 | Ka=3.0��10-8 | Ka1=4.1��10һ7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��Na2CO3�Ļ��Һ�У�������Ũ�ȵĹ�ϵ�У�c��Na+����c��CO32- ����c��CH3COO- �� | |
| B�� | ��0.1 mol•L-1 CH3COOH��Һ�еμ�NaOH��Һ����ҺpH=5����ʱ c��CH3COOH����c��CH3COO-��=9��5 | |
| C�� | ����̼�����ƹ�����뵽���Ƶ���ˮ�У�c��HC1O������ | |
| D�� | ��Ũ�Ⱦ�Ϊ1��10-3 mol•L-1��KC1��K2CrO4���Һ�еμ�1��10-3 mol•L-1 ��AgNO3��Һ��CrO42-���γɳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | CO2 | CO | |||
| A | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| B | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| C | 900 | a | b | c | d | t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���٢ۢݢ� | B�� | ���ۢݢޢ� | C�� | ���ݢޢ� | D�� | ���ۢܢޢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com