
���� ��1��CH3OH��Ũ���ᷴӦ����CH3HS04��CH3HS04����CH3OH������Ӧ���� CH3OCH3������Ԫ���غ���д��ѧ����ʽ��
��2���״��ϳɷ�Ӧ����CO��g��+2H2��g���TCH3OH��g����H1=-90.7kJ•moL-1
�ϳɶ����ѵķ�Ӧ����2CH3OH��g���TCH3OCH3��g��+H2O��g����H2=-23.5kJ•moL-1
ú���任��Ӧ����CO��g��+H20��g���TC02��g��+H2��g����H3=-41.2kJ•moL-1��
���ݸ�˹���ɢ١�2+��+�ۼ���ɵ�CO��H2ֱ�Ӻϳɶ����ѵķ�Ӧ������ֱ��ˮ�Ϸ�����������������
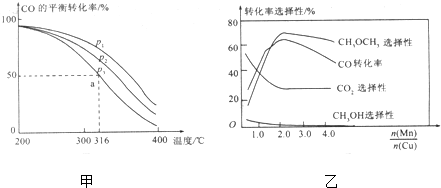
��3���ٸ÷�Ӧ������Ϊ�����С�ķ�����ѹǿ��COת���ʵ�Ӱ�������
����P3��316��ʱ��COת����Ϊ50%���ݴ˿ɼ����ƽ��ʱ�����ʵ�Ũ�ȣ���������ƽ�ⳣ����
������P3��316��ʱ����ʼʱ$\frac{n��H{\;}_{2}��}{n��CO��}$=2��1�����൱����ԭ���Ļ���������������������
��4������ͼ�����ɶ����ѵ����ֵ������
��5�����������£�������ʧ�������ɶ�����̼��
��� �⣺��1��CH3OH��Ũ���ᷴӦ����CH3HS04����ѧ����ʽΪCH3OH+H2SO4=CH3HS04+H2O��CH3HS04����CH3OH������Ӧ���� CH3OCH3����ѧ����ʽΪCH3HS04+CH3OH��CH3OCH3+H2SO4��
�ʴ�Ϊ��CH3OH+H2SO4=CH3HS04+H2O��CH3HS04+CH3OH��CH3OCH3+H2SO4��
��2����֪���״��ϳɷ�Ӧ����CO��g��+2H2��g���TCH3OH��g����H1=-90.7kJ•moL-1
�ϳɶ����ѵķ�Ӧ����2CH3OH��g���TCH3OCH3��g��+H2O��g����H2=-23.5kJ•moL-1
ú���任��Ӧ����CO��g��+H20��g���TC02��g��+H2��g����H3=-41.2kJ•moL-1��
���ݸ�˹���ɢ١�2+��+�ۼ���ɵ�CO��H2ֱ�Ӻϳɶ����ѵķ�Ӧ��3CO��g��+3H2 ��g���TCH3OCH3��g��+C02 ��g����H=��=-90.7��2-23.5-41.2��kJ/mol=-246.1kJ/mol����ϩֱ��ˮ�����з�Ӧ��������ֱ��ˮ�Ϸ��������������������Բ��������ḯʴ�豸�����⣬
�ʴ�Ϊ��-246.1kJ/mol�����������ḯʴ�豸�����⣻
��3���ٸ÷�Ӧ������Ϊ�����С�ķ�������ѹǿCO��ת������������P1��P2��P3��
�ʴ�Ϊ��P1��P2��P3��
����P3��316��ʱ��COת����Ϊ50%�����ݷ���ʽ3CO��g��+3H2 ��g���TCH3OCH3��g��+C02��֪��ƽ��ʱCO�����ʵ�����Ũ��Ϊ1mol/L��H2�����ʵ�����Ũ��Ϊ1mol/L��C02�����ʵ�����Ũ��Ϊ$\frac{1}{3}$mol/L��CH3OCH3�����ʵ�����Ũ��Ϊ$\frac{1}{3}$mol/L������ƽ�ⳣ��K=$\frac{\frac{1}{3}��\frac{1}{3}}{1��1}$=$\frac{1}{9}$��
�ʴ�Ϊ��$\frac{1}{9}$��
������P3��316��ʱ����ʼʱ$\frac{n��H{\;}_{2}��}{n��CO��}$=2��1������������������������������Ũ�ȣ�ƽ�����ƣ�CO��ת������������COת���ʴ���50%��
�ʴ�Ϊ������
��4����ͼ��֪��������$\frac{n��Mn��}{n��Cu��}$ԼΪ2ʱ��CO��ת����������ɶ����ѵ���ࣻ
�ʴ�Ϊ��2��
��5�����������£��������ڸ���ʧ�������ɶ�����̼����缫��ӦʽΪ��CH3OCH3-12e-+3H2O=2CO2��+12H+��
�ʴ�Ϊ��CH3OCH3-12e-+3H2O=2CO2��+12H+��
���� ���⿼�������Ȼ�ѧ����ʽ��˹���ɼ���Ӧ�ã�Ӱ��ƽ������ط����жϣ�ԭ���ԭ���ķ���Ӧ�ã���Ŀ�漰��֪ʶ��϶࣬�ۺ��Խ�ǿ����Ŀ���еȣ�


 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | Tl��T2����H��O | B�� | Tl��T2����H��O | C�� | Pl��P2��a+b��c | D�� | Pl��P2��a+b��c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��SO42-��Cl-��NO3- | B�� | Na+��HCO3-��Ca2+��Cl- | ||
| C�� | Na+��Cl-��NO3-��Fe3+ | D�� | K+��Cl��CO32-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ֱ����Դ�������ʽ�������ڵ绯ѧ���������������������� | |
| B�� | ��ѧ������ʹ������������ʶ���ʵ������磬�������ǽ���ɨ������������Ӧ��SMT�������ԡ�������Խ��Խϸ�Ľṹ����ʵ�ֶ�ԭ�ӻ���ӵIJ��� | |
| C�� | ����ͨ���ñ�ȼ���Ȼ���ֵ������ȼ��ȼ�շų������Ĵ�С��ij���ʵ���ֵԽ�������ȼ����Խ�� | |
| D�� | ����β����ת��װ�ÿɽ�β���е�NO��CO���к�����ת��ΪN2��CO2����װ���еĴ����ɽ���NO��CO��Ӧ�Ļ�ܣ��ӿ�÷�Ӧ�����ʣ�ͬʱ����߸÷�Ӧ��ƽ��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ͼ1��ʾ��ѧ��Ӧ2S02��g��+O2��g��?2S03��g���������仯���÷�Ӧ�ġ�H=AһBkJ/mol | |
| B�� | ͼ2��ʾ������������ʱ����Ӧ2A��g��+B��g��?c��g��+D��g���ڲ�ͬѹǿ����ʱ��ı仯 | |
| C�� | ͼ3��ʾ�����Ũ�Ⱦ���ͬ��HCl��CH3COOH������Һ�У��ֱ����������п������H2�������ʱ��ı仯����a��ʾCH3COOH��Һ | |
| D�� | ͼ4��ʾ100 ml O��1 mol•L-Na2C03��NaHC03������Һ�У��ֱ���εμ�0.1 mol•L-1HCl������CO2 ���������������ı仯����b��ʾNaHCO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
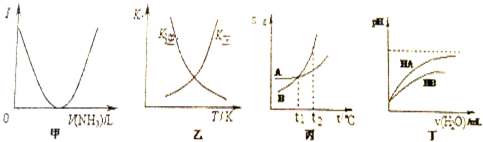
| A�� | �ɱ�ʾ������Һ��ͨ�백����������������Һ�����ԣ�I���ı仯 | |
| B�� | �ҿɱ�ʾ�ں����ܱ������з�Ӧ��2SO2��g��+O2��g��?2SO3��g����H��0����ƽ�ⳣ��K����K�����¶ȵı仯 | |
| C�� | ����ʾA��B�����ʵ��ܽ�����¶ȱ仯�������t1��ʱA��B�ı�����Һ�ֱ�������t2��ʱ�����ʵ���������B��A | |
| D�� | ����ʾ�����£�ϡ��pH��ͬ��HA��HB�������ϡ��Һʱ����ҺpH���ˮ���ı仯����HA�����Ա�HB������ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com