
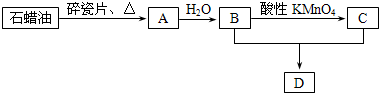
(1)��֪��101 kPaʱ��29 g������������������ȫȼ������Һ̬ˮ�Ͷ�����̼���壬�ų�QkJ����������д��������ȫȼ�յ��Ȼ�ѧ����ʽ___________________________��
(2)�Զ���Ϊȼ�ϡ�����Ϊ��������ѡ�þ��д����ú͵������ܵ�ϡ����������Ϊ�缫�������ڵ�K2CO3(���в���O2-��![]() )Ϊ��������ȼ�ϵ�ء�
)Ϊ��������ȼ�ϵ�ء�
������Ӧ��2C4H10+26![]() -52e-====34CO2+10H2O
-52e-====34CO2+10H2O
������Ӧ��____________________________________��
Ϊ��ʹȼ�ϵ�س�ʱ���ȶ����У���صĵ�������Ӧ�����ȶ���Ϊ�ˣ�������ͨ��Ŀ����м���һ�����ʣ������������_________��������___________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| Cu |
| �� |
| Cu |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�
��1���ҹ�ʢ��ϡ����17��ϡ��Ԫ�ص����ʷdz��ӽ������л���ȡ��������ϡ��Ԫ����һ����Ҫ�ļ�����������A�������е�һ�֣���ṹ��ʽ���£�
��������ѧ��֪ʶ�ж�A���ڣ� ��
A������ B������ C��ȩ�� D������
��2�����ڱ��ĺ�̼������Ȳ��ͬ��������Ϊ����һ�ֲ���������д������һ�ֺ���������֧����ͬ���칹��Ľṹ��ʽ______________________________________��
��3��ϩ�������Ը��������Һ��Ӧ���������������µĶ�Ӧ��ϵ��
��֪ijϩ���Ļ�ѧʽΪC5 H10���������Ը��������Һ��Ӧ��õ��IJ�����Ϊ������̼�Ͷ�ͪ�����ϩ���Ľṹ��ʽ�� ��
��4��������������±�ص��ʣ�±���ⷢ�������ӳɷ�Ӧ���绷������HBr��һ�������·�Ӧ���仯ѧ����ʽΪ (����ע����Ӧ����)��
��5�����������
��6��Ϊ��֤��Ȳ����ķ�Ӧ�Ǽӳɶ�����ȡ����ijͬѧ�������pH��ֽ�����Է�Ӧ����Һ�����ԣ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʵ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣�
��1���ҹ�ʢ��ϡ����17��ϡ��Ԫ�ص����ʷdz��ӽ������л���ȡ��������ϡ��Ԫ����һ����Ҫ�ļ�����������A�������е�һ�֣���ṹ��ʽ���£�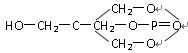
��������ѧ��֪ʶ�ж�A���ڣ� ��
| A������ | B������ | C��ȩ�� | D������ |
 ϵ��
ϵ��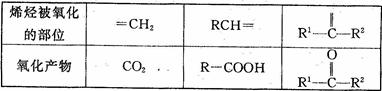
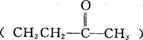 �����ϩ���Ľṹ��ʽ�� ��
�����ϩ���Ľṹ��ʽ�� ��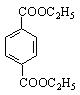
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʵ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣�
��1���ҹ�ʢ��ϡ����17��ϡ��Ԫ�ص����ʷdz��ӽ������л���ȡ��������ϡ��Ԫ����һ����Ҫ�ļ�����������A�������е�һ�֣���ṹ��ʽ���£�
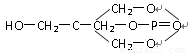
��������ѧ��֪ʶ�ж�A���ڣ� ��
A������ B������ C��ȩ�� D������
��2�����ڱ��ĺ�̼������Ȳ��ͬ��������Ϊ����һ�ֲ���������д������һ�ֺ���������֧����ͬ���칹��Ľṹ��ʽ______________________________________��
��3��ϩ�������Ը��������Һ��Ӧ���������������µĶ�Ӧ��ϵ��

��֪ijϩ���Ļ�ѧʽΪC5 H10
���������Ը��������Һ��Ӧ��õ��IJ�����Ϊ������̼�Ͷ�ͪ �����ϩ���Ľṹ��ʽ��
��
�����ϩ���Ľṹ��ʽ��
��
��4��������������±�ص��ʣ�±���ⷢ�������ӳɷ�Ӧ���绷������HBr��һ�������·�Ӧ���仯ѧ����ʽΪ (����ע����Ӧ����)��
��5�����������

��6��Ϊ��֤��Ȳ����ķ�Ӧ�Ǽӳɶ�����ȡ����ijͬѧ�������pH��ֽ�����Է�Ӧ����Һ�����ԣ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com