
£Ø19·Ö£©Ä³ĪļÖŹA¾ĻĀĶ¼ĖłŹ¾µÄ¹ż³Ģ×Ŗ»ÆĪŖŗ¬ŃõĖįD£¬ŅŃÖŖDĪŖĒæĖį£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōA³£ĪĀĻĀĪŖµ»ĘÉ«¹ĢĢåµ„ÖŹ£¬BŹĒÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĪŽÉ«ĘųĢ唣
¢Ł×é³ÉAµÄŌŖĖŲŌŚÖÜĘŚ±ķĪ»ÖĆĪŖ””””””””””””””””””””””£»
¶ŌÓ¦Ąė×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ””””””””””””””””””””””””””””””””””
¢ŚŠ“³öB”śC·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””””””
¢ŪČō½«BĶØČėĘ·ŗģČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””ĢåĻÖĮĖBµÄ
ŠŌ£»Čō½«BĶØČė×ĻÉ«µÄŹÆČļČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””””””””””””””
ĢåĻÖĮĖBµÄĖ®ČÜŅŗµÄ ŠŌ”£
¢ÜDµÄÅØČÜŅŗŌŚ¼ÓČČĢõ¼žĻĀæÉÓėĶ·“Ó¦²¢Éś³ÉBĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””£
£Ø2£©ČōAŌŚ³£ĪĀĻĀĪŖĘųĢ壬ĒŅÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»CŹĒŗģ×ŲÉ«ĘųĢ唣
¢ŁA”¢CµÄ»ÆѧŹ½·Ö±šŹĒ£ŗA””””””””””””””£¬C
¢ŚŠ“³öA”śB·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””
¢ŪČō½«AĶØČėµĪÓŠ·ÓĢŖµÄĖ®ÖŠ£¬ĻÖĻó”””””””” ĒėÓĆ ·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
£»
¢ÜDµÄÅØČÜŅŗæÉÓėĶ·“Ó¦²¢Éś³ÉCĘųĢ壬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””””””””””””””£
(¹²19·Ö)£Ø2£©¢Ł””””µŚČżÖÜĘŚVIA×å””£»”” ”””” (ø÷1·Ö)
”””” (ø÷1·Ö)
¢Ś””2SO2+O2 2SO3(2·Ö)
2SO3(2·Ö)
””””¢Ū””Ę·ŗģČÜŅŗĶŹÉ«£»ĘÆ°×£»ČÜŅŗ±äŗģ£»Ėį(ø÷1·Ö)
¢Ü””Cu+2 H2SO4£ØÅØ£©”÷Cu SO4 +SO2”ü+2H2O (2·Ö)
””£Ø2£©¢Ł””NH3£¬ NO2(ø÷1·Ö) ¢Ś4NH3+5O2== 4NO+6H2O (2·Ö)
””””¢ŪČÜŅŗ±äŗģ(1·Ö)””£»NH3+H2O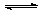 NH3”¤ H2O
NH3”¤ H2O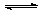 NH4++OH- (2·Ö)
NH4++OH- (2·Ö)
¢ÜCu£«4HNO3(ÅØ)£½Cu(NO3)2£«2NO2”ü£«2H2O (2·Ö)
½āĪö



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø19·Ö£© ijĪļÖŹA¾ĻĀĶ¼ĖłŹ¾µÄ¹ż³Ģ×Ŗ»ÆĪŖŗ¬ŃõĖįD£¬ŅŃÖŖDĪŖĒæĖį£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōA³£ĪĀĻĀĪŖµ»ĘÉ«¹ĢĢåµ„ÖŹ£¬BŹĒÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĪŽÉ«ĘųĢ唣
¢Ł×é³ÉAµÄŌŖĖŲŌŚÖÜĘŚ±ķĪ»ÖĆĪŖ””””””””””””””””””””””£»
¶ŌÓ¦Ąė×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ””””””””””””””””””””””””””””””””””
¢ŚŠ“³öB”śC·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””””””
¢ŪČō½«BĶØČėĘ·ŗģČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””ĢåĻÖĮĖBµÄ
ŠŌ£»Čō½«BĶØČė×ĻÉ«µÄŹÆČļČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””””””””””””””
ĢåĻÖĮĖBµÄĖ®ČÜŅŗµÄ ŠŌ”£
¢ÜDµÄÅØČÜŅŗŌŚ¼ÓČČĢõ¼žĻĀæÉÓėĶ·“Ó¦²¢Éś³ÉBĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””£
£Ø2£©ČōAŌŚ³£ĪĀĻĀĪŖĘųĢ壬ĒŅÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»CŹĒŗģ×ŲÉ«ĘųĢ唣
¢ŁA”¢CµÄ»ÆѧŹ½·Ö±šŹĒ£ŗA””””””””””””””£¬C
¢ŚŠ“³öA”śB·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””
¢ŪČō½«AĶØČėµĪÓŠ·ÓĢŖµÄĖ®ÖŠ£¬ĻÖĻó”””””””” ĒėÓĆ·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
£»
¢ÜDµÄÅØČÜŅŗæÉÓėĶ·“Ó¦²¢Éś³ÉCĘųĢ壬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÕć½Ź”øßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
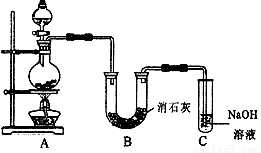
(20·Ö)ĀČĘųŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬×ŌĄ“Ė®µÄĻū¶¾”¢Å©Ņ©µÄÉś²ś”¢Ņ©ĪļµÄŗĻ³ÉµČ¶¼ŠčŅŖÓƵ½ĀČĘų”£¹¤ŅµÉĻĶس£²ÉÓƵē½ā·ØÖĘĀČĘų£ŗ¹Ū²ģĻĀĶ¼£¬»Ų“š£ŗ
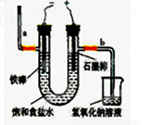
¢ń(1)Čō±„ŗĶŹ³ŃĪĖ®ÖŠŗ¬ÓŠ·ÓĢŖ£¬Ķصēŗó_____(Ģīa»ņb)²ąĻȱäŗģ”£
(2)µē½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________
¢ņÄ³Ń§ÉśÉč¼ĘČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬ĄūÓĆĀČĘųÓė³±ŹŖµÄĻūŹÆ»Ņ·“Ó¦ÖĘȔɣĮæĘÆ°×·Ū£ØÕāŹĒŅ»øö·ÅČČ·“Ó¦£©£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1) ŌŚA×°ÖĆÖŠÓĆ¹ĢĢ嶞Ńõ»ÆĆĢÓėÅØŃĪĖįŌŚ¼ÓČČĢõ¼žĻĀÖĘČ”ĀČĘų£¬·“Ó¦»Æѧ·½³ĢŹ½ĪŖ £¬ČōŌŚ±ź×¼×“Ģ¬ĻĀŹÕ¼Æµ½22.4ÉżµÄĀČĘų£¬Ōņ±»Ńõ»ÆµÄHClµÄĪļÖŹµÄĮæŹĒ ”£
(2) ĘÆ°×·Ū½«ŌŚUŠĪ¹ÜÖŠ²śÉś£¬Ęä»Æѧ·½³ĢŹ½ŹĒ ”£
(3) C×°ÖƵÄ×÷ÓĆŹĒ ”£
(4) “ĖŹµŃéĖłµĆĘÆ°×·ŪµÄÓŠŠ§³É·ÖĘ«µĶ,øĆѧɜ¾·ÖĪö²¢²éŌÄ׏ĮĻ·¢ĻÖ£¬Ö÷ŅŖŌŅņŹĒŌŚUŠĪ¹ÜÖŠ»¹“ęŌŚĮ½øöø±·“Ó¦”£
¢ŁĪĀ¶Č½Ļøߏ±ĀČĘųÓėĻūŹÆ»Ņ·“Ӧɜ³ÉCa(ClO3)2£¬ĪŖ±ÜĆā“Ėø±·“Ó¦µÄ·¢Éś£¬æɲÉČ”µÄ“ėŹ©ŹĒ ”£
¢ŚŹŌÅŠ¶ĻĮķŅ»øöø±·“Ó¦(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾) ”£ĪŖ±ÜĆā“Ėø±·“Ó¦µÄ·¢Éś£¬æɽ«×°ÖĆ×÷ŗĪøĽų ”£
(5)¼ŅĶ„ÖŠŹ¹ÓĆĘÆ°×·ŪŹ±£¬ĪŖĮĖŌöĒæĘÆ°×ÄÜĮ¦£¬æɼÓČėÉŁĮæµÄĪļÖŹŹĒ ”£
A£®Ź³ŃĪ B.Ź³“× C.ÉÕ¼ī D.“æ¼ī
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģø£½ØŹ”ø£ÖŻŹŠ°ĖĻŲ£ØŹŠ£©øßŅ»ĻĀѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø19·Ö£© ijĪļÖŹA¾ĻĀĶ¼ĖłŹ¾µÄ¹ż³Ģ×Ŗ»ÆĪŖŗ¬ŃõĖįD£¬ŅŃÖŖDĪŖĒæĖį£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōA³£ĪĀĻĀĪŖµ»ĘÉ«¹ĢĢåµ„ÖŹ£¬BŹĒÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĪŽÉ«ĘųĢ唣
¢Ł×é³ÉAµÄŌŖĖŲŌŚÖÜĘŚ±ķĪ»ÖĆĪŖ””””””””””””””””””””””£»
¶ŌÓ¦Ąė×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ””””””””””””””””””””””””””””””””””
¢ŚŠ“³öB”śC·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””””””
¢ŪČō½«BĶØČėĘ·ŗģČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””ĢåĻÖĮĖBµÄ
ŠŌ£»Čō½«BĶØČė×ĻÉ«µÄŹÆČļČÜŅŗÖŠ£¬ĻÖĻó””””””””””””””””””””””””””””””””””
ĢåĻÖĮĖBµÄĖ®ČÜŅŗµÄ ŠŌ”£
¢ÜDµÄÅØČÜŅŗŌŚ¼ÓČČĢõ¼žĻĀæÉÓėĶ·“Ó¦²¢Éś³ÉBĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””£
£Ø2£©ČōAŌŚ³£ĪĀĻĀĪŖĘųĢ壬ĒŅÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»CŹĒŗģ×ŲÉ«ĘųĢ唣
¢ŁA”¢CµÄ»ÆѧŹ½·Ö±šŹĒ£ŗA””””””””””””””£¬C
¢ŚŠ“³öA”śB·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ””””””””””””””””””””””””””””””
¢ŪČō½«AĶØČėµĪÓŠ·ÓĢŖµÄĖ®ÖŠ£¬ĻÖĻó”””””””” ĒėÓĆ·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
£»
¢ÜDµÄÅØČÜŅŗæÉÓėĶ·“Ó¦²¢Éś³ÉCĘųĢ壬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
”””””””””””””””””””””””””””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”ĢØ֯֊ѧøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(20·Ö)ĀČĘųŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬×ŌĄ“Ė®µÄĻū¶¾”¢Å©Ņ©µÄÉś²ś”¢Ņ©ĪļµÄŗĻ³ÉµČ¶¼ŠčŅŖÓƵ½ĀČĘų”£¹¤ŅµÉĻĶس£²ÉÓƵē½ā·ØÖĘĀČĘų£ŗ¹Ū²ģĻĀĶ¼£¬»Ų“š£ŗ
¢ń(1)Čō±„ŗĶŹ³ŃĪĖ®ÖŠŗ¬ÓŠ·ÓĢŖ£¬Ķصēŗó_____(Ģīa»ņb)²ąĻȱäŗģ”£
(2)µē½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________
¢ņÄ³Ń§ÉśÉč¼ĘČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬ĄūÓĆĀČĘųÓė³±ŹŖµÄĻūŹÆ»Ņ·“Ó¦ÖĘȔɣĮæĘÆ°×·Ū£ØÕāŹĒŅ»øö·ÅČČ·“Ó¦£©£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ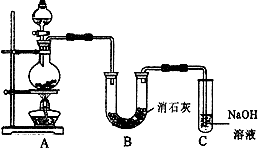
(1) ŌŚA×°ÖĆÖŠÓĆ¹ĢĢ嶞Ńõ»ÆĆĢÓėÅØŃĪĖįŌŚ¼ÓČČĢõ¼žĻĀÖĘČ”ĀČĘų£¬·“Ó¦»Æѧ·½³ĢŹ½ĪŖ  £¬ČōŌŚ±ź×¼×“Ģ¬ĻĀŹÕ¼Æµ½22.4ÉżµÄĀČĘų£¬Ōņ±»Ńõ»ÆµÄHClµÄĪļÖŹµÄĮæŹĒ ”£
£¬ČōŌŚ±ź×¼×“Ģ¬ĻĀŹÕ¼Æµ½22.4ÉżµÄĀČĘų£¬Ōņ±»Ńõ»ÆµÄHClµÄĪļÖŹµÄĮæŹĒ ”£
(2) ĘÆ°×·Ū½«ŌŚUŠĪ¹ÜÖŠ²śÉś£¬Ęä»Æѧ·½³ĢŹ½ŹĒ ”£
(3) C×°ÖƵÄ×÷ÓĆŹĒ ”£
(4) “ĖŹµŃéĖłµĆĘÆ°×·ŪµÄÓŠŠ§³É·ÖĘ«µĶ,øĆѧɜ¾·ÖĪö²¢²éŌÄ׏ĮĻ·¢ĻÖ£¬Ö÷ŅŖŌŅņŹĒŌŚUŠĪ¹ÜÖŠ»¹“ęŌŚĮ½øöø±·“Ó¦”£
¢ŁĪĀ¶Č½Ļøߏ±ĀČĘųÓėĻūŹÆ»Ņ·“Ӧɜ³ÉCa(ClO3) 2£¬ĪŖ±ÜĆā“Ėø±·“Ó¦µÄ·¢Éś£¬æɲÉČ”µÄ“ėŹ©ŹĒ ”£
2£¬ĪŖ±ÜĆā“Ėø±·“Ó¦µÄ·¢Éś£¬æɲÉČ”µÄ“ėŹ©ŹĒ ”£
¢ŚŹŌÅŠ¶ĻĮķŅ»øöø±·“Ó¦(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾) ”£ĪŖ±ÜĆā“Ėø±·“Ó¦µÄ·¢Éś£¬æɽ«×°ÖĆ×÷ŗĪøĽų ”£
(5)¼ŅĶ„ÖŠŹ¹ÓĆĘÆ°×·ŪŹ±£¬ĪŖĮĖŌöĒæĘÆ°×ÄÜĮ¦£¬æɼÓČėÉŁĮæµÄĪļÖŹŹĒ ”£
| A£®Ź³ŃĪ | B£®Ź³“× | C£®ÉÕ¼ī | D£®“æ¼ī |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com