
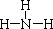 ��д����������������������Һ��Ӧ�Ļ�ѧ����ʽΪAlN+NaOH+H2O=NaAlO2+NH3������֤��Һ���в��������ӵ�ʵ�鷽��Ϊȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2��
��д����������������������Һ��Ӧ�Ļ�ѧ����ʽΪAlN+NaOH+H2O=NaAlO2+NH3������֤��Һ���в��������ӵ�ʵ�鷽��Ϊȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2�� ���� �����Ʊ����ֲ��ϵ���ʼԭ�ϣ�Ҳ�����������Ҫ�ɷ֣�����ΪAl2O3���Ʊ�ʱ��ȡ̼�Ȼ�ԭ����������������̿�۰�һ��������ϣ�������N2�����м��ȵ�һ���¶ȼ��ɵõ��Ϳ�ȼ�������������ԭ�Ӹ���֮�Ⱦ�Ϊ1��1������Al2O3+N2+3C$\frac{\underline{\;\;��\;\;}}{\;}$2AlN+3CO�����ΪAlN����ΪCO��8.2g�ף�AlN��������������������Һ��Ͽ��Եõ���ɫ��Һ������������״����4.48L�����죬��Ϊ������n���ף�=n���죩=0.2mol���Դ������
��� �⣺�����Ʊ����ֲ��ϵ���ʼԭ�ϣ�Ҳ�����������Ҫ�ɷ֣�����ΪAl2O3���Ʊ�ʱ��ȡ̼�Ȼ�ԭ����������������̿�۰�һ��������ϣ�������N2�����м��ȵ�һ���¶ȼ��ɵõ��Ϳ�ȼ�������������ԭ�Ӹ���֮�Ⱦ�Ϊ1��1������Al2O3+N2+3C$\frac{\underline{\;\;��\;\;}}{\;}$2AlN+3CO�����ΪAlN����ΪCO��8.2g�ף�AlN��������������������Һ��Ͽ��Եõ���ɫ��Һ������������״����4.48L�����죬��Ϊ����
��1����ΪAlN���Թ��ۼ����������ʣ��������¡�������������Ժõ��������ʣ���Ϊԭ�Ӿ��壬̼�Ȼ�ԭ�������ƼĻ�ѧ����ʽΪAl2O3+3C+N2$\frac{\underline{\;\;��\;\;}}{\;}$2AlN+3CO��
�ʴ�Ϊ��ԭ�Ӿ��壻Al2O3+3C+N2$\frac{\underline{\;\;��\;\;}}{\;}$2AlN+3CO��
��2����Al2O3+3C+N2$\frac{\underline{\;\;��\;\;}}{\;}$2AlN+3CO��֪����Ӧ���й������ʣ�࣬��������Al4C3���Ʊ��Ĺ����п��ܺ��е�������Al2O3��C��Al4C3�ȣ�
�ʴ�Ϊ��Al2O3��C��Al4C3�ȣ�
��3����ĽṹʽΪ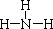 ����������������������Һ��Ӧ�Ļ�ѧ����ʽΪAlN+NaOH+H2O=NaAlO2+NH3������֤��Һ�������ʵ�ʵ�鷽��Ϊȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2��
����������������������Һ��Ӧ�Ļ�ѧ����ʽΪAlN+NaOH+H2O=NaAlO2+NH3������֤��Һ�������ʵ�ʵ�鷽��Ϊȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2��
�ʴ�Ϊ�� ��AlN+NaOH+H2O=NaAlO2+NH3����ȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2��
��AlN+NaOH+H2O=NaAlO2+NH3����ȡ������Һ�����Թ��У���μ���ϡ���ᣬ���Ȳ�����ɫ��������ʧ��˵��ΪNaAlO2��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬���������Ʊ����ֲ��ϵ���ʼԭ�ϣ�Ҳ�����������Ҫ�ɷ����ƶ���Ϊ����ͻ�ƿڣ���ȷ�����ķ�Ӧ�����ʵ������ƶϼ��ɣ����ط������ƶ������Ŀ��飬��Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Ӧ�У�����1molP�μӷ�Ӧʱ����Ӧת�Ƶ�������Ϊ5NA | |
| B�� | �ڷ�Ӧ��2���У�������3mol����ʱ������ԭ��PΪ1mol | |
| C�� | ������������Ӧ�У�P������ԭ�� | |
| D�� | ������������Ӧ�У���ԭ���ﶼ��H3PO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ���ȳʺ�ɫ����Һ�У�Fe2+��Na+��ClO-��SO42- | |
| B�� | ��PH=3����Һ�У�Mg2+��Al3+��NO3-��Cl- | |
| C�� | ��ˮ�������c��H+��=10-13mol•L-1����Һ�У�Na+��SO32-��AlO2-��Br- | |
| D�� | �������ۺ����������������Һ�У�NH4+��K+��NO3-��MnO4- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��PH�İ�ˮ��KOH��Һ��Ba��OH��2��Һ�У�c��NH4+��=c��K+��=c��Ba2+�� | |
| B�� | ������������ʵ���Ũ�ȵ�NaCl��NaClO��Һ������������N����N��NaCl����N��NaClO�� | |
| C�� | ��10mol0.1mol•L-1Na2CO3��Һ�μӵ�10mL0.1mol•L-1�����У�c��Na+����c��Cl-����c��HCO3-����c��CO32-�� | |
| D�� | �����£���0.1mol•L-1�Ĵ�������Һ��ͨ���Ȼ������壬ʹ��Һ��pH=7����c��CH3COOH��=c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ����Һ����ɫ����Һ�У�K+��Na+��HSO3-��ClO- | |
| B�� | 0.1mol/L��Fe��NO3��3��Һ�У�Na+��H+��Cl-��I- | |
| C�� | 0.1mol/L��NaAlO2��Һ�У�K+��H+��NO3-��SO42- | |
| D�� | ��ˮ���������c��H+��=1��10-13mol/L����Һ�У�Na+��Ba2+��NO3-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ��ת�Ƶ��ӵ�����Ϊ0.3mol | |
| B�� | ��Ӧ�����У�������������ʵ���Ϊ0.3mol | |
| C�� | ������ȫʱ����NaOH��Һ�����Ϊ100mL | |
| D�� | ��ʼ����Ͻ����������Ϊ3.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
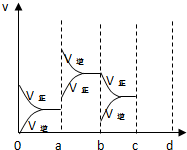 ̼���𡢵������ĵ��ʼ��仯�����ڹ�ũҵ����������������Ҫ�����ã�
̼���𡢵������ĵ��ʼ��仯�����ڹ�ũҵ����������������Ҫ�����ã�| ʱ��/min Ũ��/mol/L | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
| N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
| CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com