
| 40 |
| 36.5 |
| 8g |
| X |
| 7.3g |
| 10% |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��MnO2������Ũ���ᷴӦ��ȡCl2��MnO2+4HCl
| ||||
| B��AlCl3��Һ�еμ�Ũ��ˮ��������Al3++4NH3?H2O�TAlO2-+4NH4++2H2O | ||||
| C���Ȼ�������Һ�м������3Fe2++4H++NO3-�T3Fe3++2H2O+NO�� | ||||
| D��NH4HCO3���ڹ�����NaOH��Һ�У�HCO3-+OH-�TCO32-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
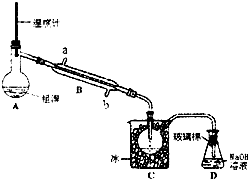 �屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ590C������ˮ���ж��Ժ�ǿ���ԣ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
�屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ590C������ˮ���ж��Ժ�ǿ���ԣ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����-KI��ֽ�������� | ����ƿ�ռ����� | ����ƿ |
| Ӧ����NaOH������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| �ձ�����������ҩ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
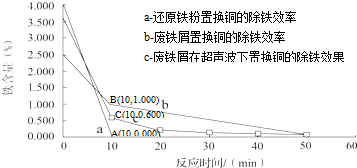
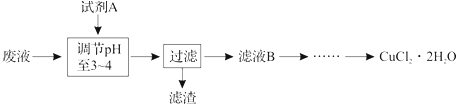
| ������ | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ���� | 2.3 | 7.5 | 4.7 |
| ��ȫ���� | 4.1 | 9.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 200 | 300 | 400 | 500 | 600 |
| ������/% | 89.9 | 71.0 | 47.0 | 26.4 | 13.8 |
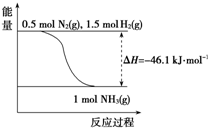
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com