
ij�߶���ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ�������¡��Ը����±��е�ʵ������ش��������⣺
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
(1)ʵ��1��2��Al�����ĵ缫�Ƿ���ͬ��________(��ǡ���)��
(2)ʵ��3�еĵ缫��Ӧʽ������______________________������______________________________������ܷ�Ӧ����ʽ______________________��
(3)ʵ��4��Al��________������缫��Ӧʽ��____________________________���жϵ缫��������_____________________________________________________________��
(4)����ʵ��5�е�����ָ��ƫ��Al��ԭ��
�𰸡�(1)��
(2)Al����Al3����3e����2H����2e������H2��
2Al��6H��===2Al3����3H2��
(3)����Al��4OH������[Al(OH)4]����3e��
��ΪAl����NaOH��Һ��Ӧ����Mg����Ӧ
(4)ʵ��5������Al��Ũ�����з����ۻ�������ZnΪ������
������һ������£��ϻ��õĽ�����ԭ��صĸ���������ʵ��1��2�Ľ�����ɵó�1��Al��������2��Al������������NaOH��Һ�У�Al��Ӧ��Mg����Ӧ��Al����������Ũ����������Al�����ۻ���Zn��������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�


ʵ�鲽�裺
��A�м���4.4 g�����촼��6.0 g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50 min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g���ش��������⣺
(1)����B��������________________��
(2)��ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����________________�� �ڶ���ˮϴ����ҪĿ����______________________��
(3)��ϴ�ӡ��� Һ�����У�Ӧ�����Ȼ���ã����ֲ��________(��ѡ����ĸ)��
Һ�����У�Ӧ�����Ȼ���ã����ֲ��________(��ѡ����ĸ)��
a��ֱ�ӽ������������ӷ�Һ©���Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©���¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
(4)��ʵ���м�����������Ŀ����_________________________��
(5)ʵ���м���������ˮMgSO4 ��Ŀ����____________________��
��Ŀ����____________________��
(6)����������У�����ѡ��װ����ȷ����_______ _(��ѡ����ĸ)��
_(��ѡ����ĸ)��

(7)��ʵ��IJ�����________(��ѡ����ĸ)
A��30%������B��40%������C��60%������D��90%
(8)�ڽ����������ʱ������130 �濪ʼ�ռ���֣���ʹʵ��IJ���ƫ________(��ߡ��� ���͡�)����ԭ����
���͡�)����ԭ����
__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��R��X��T��Z��Q��Ԫ�����ڱ��е����λ�����±���ʾ�� ����R�����ڰ�����H2���һ��ϲ�������ը���������ж���ȷ����
 A���ǽ����ԣ�Z<T<X
A���ǽ����ԣ�Z<T<X
B��R��Q�ĵ��������26
C����̬�⻯���ȶ��ԣ�R <T<Q
D������������ˮ��������ԣ�T>Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH ��Cl����Mg2����Ba2����CO
��Cl����Mg2����Ba2����CO ��SO
��SO ����ȡ����100 mL��Һ��������ʵ�飺
����ȡ����100 mL��Һ��������ʵ�飺
(1)��һ�ݼ���AgNO3��Һ�г���������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol��
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
��������ʵ�飬�����Ʋ���ȷ����(����)
A��K����һ������
B��100 mL��Һ�к�0.01 mol CO
C��Cl�����ܴ���
D��Ba2��һ�������ڣ�Mg2�����ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ձ����ֱ�ʢ���Ȼ�ͭ���Ȼ��غ�������������Һ������Pt���缫�������Ǵ�����һ����һ��ʱ�䣬��õ缫�����ܺ�Ϊ2.8 g����ʱ��������ɫ��������ɫ��������ʵ���֮��Ϊ(����)��������������������������������
A��4��1 B��1��1 C��4��3 D��3��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڲ�ͬ״̬�£���̬����̬����������Ӧ���Ȼ�ѧ����
ʽ������ʾ��
��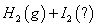 =
=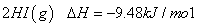
��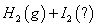 =
=
�����ж���ȷ����
A�����е�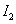 Ϊ��̬�����е�
Ϊ��̬�����е�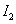 Ϊ��̬
Ϊ��̬
B���ڵķ�Ӧ���������Ȣٵķ�Ӧ����������
C���ٵIJ���ȷ�Ӧ�ڵIJ������ȶ��Ը���
D��1mol��̬������ʱ������17kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼���γɻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʡ���ش��������⣺
��1���л���M����̫������տ�ת����N��ת���������£�

 ����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
��2����֪CH3OH(l)��ȼ���� Ϊ238.6 kJ·mol��1��CH3OH(l)��
Ϊ238.6 kJ·mol��1��CH3OH(l)�� O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
��3��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��______________________________��
��4������͵�������ı������������ʡ���ʯī�����ۺͶ������Ѱ�һ����������ڸ��������գ��������ʿ������²��ϣ�4Al(s)��3TiO2(s)��3C(s)===2Al2O3(s)��3TiC(s)����H����1 176 kJ·mol��1����Ӧ�����У�ÿת��1 mol���ӷų�������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�
mCeO2 ��m-x��CeO2·xCe+xO2
��m-x��CeO2·xCe+xO2
��m-x��CeO2·xCe+xH2O+ xCO2 mCeO2+ xH2+ xCO
mCeO2+ xH2+ xCO
 ����˵������ȷ����
����˵������ȷ����
A���ù�����CeO2û������
B���ù���ʵ����̫������ѧ�ܵ�ת��
C����ͼ�С�H1=��H2+��H3
D����CO��O2���ɵļ���ȼ�ϵ�صĸ�����ӦʽΪ
CO+4OH——2e—=CO +2H2O
+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4.6 g��Ͷ�뵽95.4 gˮ�У�������Һ�����ʵ���������Ϊ(����)
A������4.6%���������������� B������8.0%
C������8.0% D����8.0%
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com