
�����й���Ԫ�ؼ��仯�����˵����������ȷ����(����)
A����ƿ��Ʊ����ᾭ��������S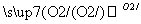 SO3
SO3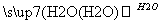 H2SO4
H2SO4
B�������������еĽ��������ﷴӦ����Ԫ���Ե��ʵ���ʽ����������
C����ȼú�м���ʯ��ʯ�ɼ���SO2�ŷţ������ķ�ӦΪ2CaCO3��2SO2��O2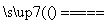 2CO2��2CaSO4
2CO2��2CaSO4
D�������е���п��(ZnS)��������ͭ��Һת��Ϊͭ��(CuS)��˵��CuS���ȶ��������л�ԭ
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���м����Լ����ܰѵ����ʵ���Ũ�ȵ�Na2CO3��NaHCO3������(����)
A��BaCl2��Һ��B������ʯ��ˮ��C��ϡ���ᡡD��pH��ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2����������ȷ����(����)
A��SO2ͨ����ˮ����Һ��ɫ�����Լ���
B�����������������ˮ���ȶ���������
C��SO2����ͨ��NaOH��Һһ���õ�Na2SO3
D��S��SO2��SiO2�������ʾ�����NaOH��Һ��Ӧ������������ijЩ�ᷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�
����һ
(1)Ũ�����Լ�ƿ���ʺ����ϵı�ǩ��________(�����)��

(2)��ͬѧȡ6.4 gͭƬ��10 mL 18 mol·L��1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ������Ұ�ɫ��������ͬѧΪ����֤���лҰ�ɫ��������Ҫ�ɷ֣��������ʵ�飺
ʵ�鲽�裺�㵹���ϲ�Һ��������ûҰ�ɫ�Ĺ����м�����������ˮ���ӱ߽��衣
ʵ������_________________________________��
ʵ����ۣ����ûҰ�ɫ����Ļ�ѧʽΪ__________��
(3)�һ��۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ������ɫ������ʧ��ԭ����(�û�ѧ����ʽ�ش�)________________________________________________________________________��
ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�࣬�Ҹ����Լ���ѧ�Ļ�ѧ֪ʶ����Ϊ�Թ��л�������ʣ�ࡣ��������Ϊ��������________________________________________________________________________��
������
(4)��ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·��2Cu��O2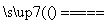 2CuO��CuO��H2SO4===CuSO4��H2O��
2CuO��CuO��H2SO4===CuSO4��H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ��Ǣ�________________________________________________________________________��
��________________________________________________________________________��
������
(5)��ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ������Һ����ɫ��д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3·5H2O)��
��.[��������]
(1)Na2S2O3·5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��.[�Ʊ���Ʒ]
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
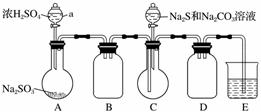
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л�����Һ��__________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.[̽���뷴˼]
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������
(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����
________________________________________________________________________��
(3)Na2S2O3·5H2O���ܽ�����¶����������������ò�Ʒͨ��________________�����ᴿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п��100 mL 18.5 mol·L��1��Ũ�����ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ���������33.6 L(��״��)������Ӧ�����Һϡ����1 L�������Һ��pH��1��������������ȷ����(����)
A����Ӧ�й�����1.8 mol H2SO4
B���������SO2��H2�������Ϊ4��1
C����Ӧ�й�����97.5 g Zn
D����Ӧ�й�ת��3 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�����ķ����У�����ԭ�Ӷ�����ͬһƽ����ǣ� ��
A�������� B����ȩ C���������ױ� D��������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺��
(1)��̬��ԭ�ӵ���Χ�����Ų�ʽΪ�� ��
(2)����������CO�γ������Ni(CO)4,д����CO��Ϊ�ȵ������һ�ַ��Ӻ�һ�����ӵĻ�ѧʽ��������������������
(3)�ܶ�����л�����Ni���¿���H2�����ӳɷ�Ӧ�����CH2=CH2����HC��CH����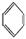 ����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� (���������),HCHO���ӵ�����ṹΪ
����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� (���������),HCHO���ӵ�����ṹΪ
����������
(4)����ͪ뿳����ڼ���Ni2+����ϡ��ˮ��,����ͪ���Ni2+��Ӧ�����ʺ�ɫ����,��ṹ����ͼ��ʾ���ýṹ��,�����ۼ��������λ��,����ͼ���ü�ͷ��ʾ����λ����
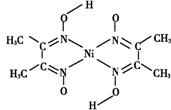
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯīϩ(ͼ��)��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ(ͼ��)��
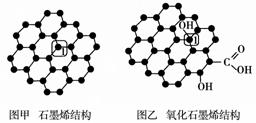
(1)ͼ���У�1��C������C�γɦҼ��ĸ���Ϊ________��
(2)ͼ���У�1��C���ӻ���ʽ��________����C������C�γɵļ���________(�>����<������)ͼ����1��C������C�γɵļ��ǡ�
(3)����ͼ����ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����________(��Ԫ�ط���)��
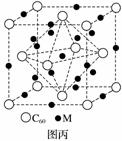
(4)ʯīϩ��ת��Ϊ����ϩ(C60)��ij����M��C60���Ʊ�һ�ֵ��³������ϣ�������ͼ����ʾ��Mԭ��λ�ھ������������ڲ����þ�����Mԭ�ӵĸ���Ϊ________���ò��ϵĻ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com