
[s1] X��Y��Z��M�����ڱ�ǰ�������е����ֳ���Ԫ�أ�ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | ԭ���������������ڲ��������2�� |
| Y | ��̬ԭ�����������Ų�Ϊ |
| Z | ��Yͬ���ڣ���һ������С��Y |
| M | ��X�γɵĺϽ�ΪĿǰ�������Ľ������� |
��1��Zλ�����ڱ��� ���ڵ� �塣Y��Z���⻯���ȶ��Խ�ǿ���� ��д��ѧʽ����
��2��X������γ�һ��ԭ�Ӹ�����Ϊ1��1�Ļ������Է�������Ϊ78���÷����д��� ��![]() ����H��X��H��Y��H��Z���ֹ��ۼ��У���������� ��
����H��X��H��Y��H��Z���ֹ��ۼ��У���������� ��
��3��MԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��4����֪�������ݣ�
 M��s��+
M��s��+![]() Z2(g) MZ(s) ��H=-272.kJ��mol-1
Z2(g) MZ(s) ��H=-272.kJ��mol-1
 2M��s��+
2M��s��+![]() Z2(g) M2Z3(s) ��H=-824.2.kJ��mol-1
Z2(g) M2Z3(s) ��H=-824.2.kJ��mol-1
MZת��ΪM2Z3���Ȼ�ѧ����ʽ��
[s1]18��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�أ���֪����X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ������Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ����
X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�أ���֪����X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ������Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ÿ��3�֣���֪��X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�ء���֪��
�� X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ����
�� Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ����
��ش�
��1��Z2Y�Ļ�ѧʽ��
��2��Z2Y2��X2Y��Ӧ�Ļ�ѧ����ʽ�� ��
��3����ͼ��ʾװ�ã�����������ʢ�����з�̪����Һ��ZW������Һ��C����C����Ϊ���ʯī�缫����ͨS1��C(��)������Һ��죬�������������������ɡ�һ��ʱ�������������Һ��δ����缫�����Ͽ�S1����ͨS2����������ָ�뷢��ƫת����ʱ��C(��)�ĵ缫������ ����д����������
��4��ͭм����ϡ���������Ӧ������ϡ�����м���X2Y2��ͭм�����ܽ⣬�÷�Ӧ������![]() ����ʽ�ǣ� ��
����ʽ�ǣ� ��
| �� �� | �ľ��� |
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ�ͷ��ʮ���и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(8��)X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�ء�
��֪����X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ������Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ����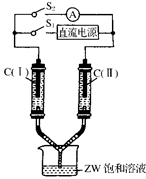
��ش�
(1)Z2Y�Ļ�ѧʽ��________________________����1�֣�
(2)Z2Y2��X2Y��Ӧ�Ļ�ѧ����ʽ��_______________________________________��2�֣�
(3)��ͼ��ʾװ�ã�����������ʢ�����з�̪��Һ��ZW������Һ��C(��)��C(��)Ϊ���ʯī�缫����ͨS1��C(��)������Һ��죬�������������������ɡ�һ��ʱ���(����������Һ��δ����缫)���Ͽ�S1����ͨS2����������ָ�뷢��ƫת����ʱ��
C(��)�ĵ缫������________(��д��������������)����1�֣�
C(��)�ĵ缫��Ӧʽ��________________________________����2�֣�
(4)ͭм����ϡ���������Ӧ������ϡ�����м���X2Y2��ͭм�����ܽ⣬�÷�Ӧ�����ӷ���ʽ��____________________________________����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���ϰ�У2010-2011ѧ������������� ���ͣ������
[s1] X��Y��Z��M�����ڱ�ǰ�������е����ֳ���Ԫ�أ�ԭ���������������������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
ԭ���������������ڲ��������2�� |
|
Y |
��̬ԭ�����������Ų�Ϊ |
|
Z |
��Yͬ���ڣ���һ������С��Y |
|
M |
��X�γɵĺϽ�ΪĿǰ�������Ľ������� |
��1��Zλ�����ڱ��� ���ڵ� �塣Y��Z���⻯���ȶ��Խ�ǿ���� ��д��ѧʽ����
��2��X������γ�һ��ԭ�Ӹ�����Ϊ1��1�Ļ������Է�������Ϊ78���÷����д���
��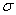 ����H��X��H��Y��H��Z���ֹ��ۼ��У����������
��
����H��X��H��Y��H��Z���ֹ��ۼ��У����������
��
��3��MԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��4����֪�������ݣ�
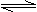 M��s��+
M��s��+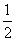 Z2(g)
MZ(s) ��H=-272.kJ��mol-1
Z2(g)
MZ(s) ��H=-272.kJ��mol-1
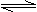 2M��s��+
2M��s��+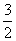 Z2(g) M2Z3(s)
��H=-824.2.kJ��mol-1
Z2(g) M2Z3(s)
��H=-824.2.kJ��mol-1
MZת��ΪM2Z3���Ȼ�ѧ����ʽ��
[s1]18��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com