
����Ŀ������㷺Ӧ����ϡ�н�����ʪ��ұ��ƯȾ��ҵ�������ӹ�����ҩƷ���л�ҩ��������������С�HCl ��������ˮ����ҵ���� HCl ��������ˮ�ķ�����ȡ���ᡣ
��1���� 12.0mol/L Ũ�������� 230mL 0.3mol/L ��ϡ���ᣬ��Ҫ��ȡŨ��������Ϊ___mL��
��2����Һϡ��������Ҫ�IJ����������ձ�������������Ͳ��____________��___________��
��3����Һϡ�����������²�����
a����ȡŨ�����һ�������ˮ�����ձ���ϡ��
b����������Ũ��������
c�����µߵ�ҡ��
d��������ˮ���̶��� 1-2cm �ط������ý�ͷ�ιܼ�����ˮ����Һ����̶�������
e����ϡ��Һת��������ƿ��ϴ���ձ��Ͳ�����������ϴ��Һת��������ƿ����
������ȷ�IJ���˳��Ϊ____________________________________________(�����)��
��4��ʵ������е����²����ᵼ������������ҺŨ�ȣ�����ƫ��������ƫС��������������
a����ȡŨ����ʱ���ӣ�______________________��
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ��______________________��
c��ʵ��ǰ������ƿ����������������ˮ��______________________��
��5����״����1L ˮ��ͨ�� aL HCl ���壬����������Һ�� HCl �Ļӷ����õ���������Һ�ܶ�Ϊ b g/mL�����ʵ���Ũ��Ϊ ______________________mol/L��
���𰸡�6.3 250mL����ƿ ��ͷ�ι� b��a��e��d��c ƫС ƫ�� ���� ![]()
��������
(1)����������Һ���ѡ������ƿ�Ĺ����������Һϡ���������ʵ����ʵ������������ҪŨ����������
(2)��������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���ݴ�����
(4)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(5)�ȸ���n=![]() �������״����aL�Ȼ�������ʵ�����Ȼ�����m=nM������Ȼ����������1Lˮ������ԼΪ1000g���Ӷ���֪��Һ�������ٸ���V=
�������״����aL�Ȼ�������ʵ�����Ȼ�����m=nM������Ȼ����������1Lˮ������ԼΪ1000g���Ӷ���֪��Һ�������ٸ���V=![]() �������Һ�������������c=
�������Һ�������������c=![]() ���������������ʵ���Ũ�ȡ�
���������������ʵ���Ũ�ȡ�
(1)��12.0mol/LŨ��������230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL����ƿ������ҪŨ�������V����������Һϡ���������ʵ����ʵ����������ã�12.0mol/L��V=0.250L��0.3mol/L�����V=0.0063L=6.3mL���ʴ�Ϊ��6.3��
(2)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ�����������Ͳ����ͷ�ιܡ��ձ���������������ƿ������230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL�������ƿ����ȱ�ٵ�������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ���ܽ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ��������ȷ��˳��Ϊ��baedc���ʴ�Ϊ��baedc��
(4)a����ȡŨ����ʱ���ӣ�������ȡ��Ũ�������ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫС���ʴ�Ϊ��ƫС��
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ��������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ʴ�Ϊ��ƫ��
c��ʵ��ǰ������ƿ����������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻�ʴ�Ϊ�����䣻
(5)��״���µ�aL�Ȼ�����������ʵ���Ϊ��n(HCl)= ![]() =
=![]() mol����HCl��������36.5g/mol��
mol����HCl��������36.5g/mol��![]() mol=
mol=![]() g��1Lˮ������ԼΪ1000g�������������Ϊ��1000g+
g��1Lˮ������ԼΪ1000g�������������Ϊ��1000g+![]() g������������Ϊ��
g��������������![]() =
=![]() mL=
mL=![]() L�����Ը���������ʵ���Ũ��Ϊ��c(HCl)=
L�����Ը���������ʵ���Ũ��Ϊ��c(HCl)= 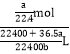 =
=![]() mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ�� ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C1O2��Ϊһ��ǿ���������ǹ����Ϲ��ϵĸ�Ч������������������ױ��ж�������NaClO2����������£���NaOH������뵽��0.1molC1O2����ˮ��ɵ�1L��Һ�С���ҺpH��������ֺ����仯������ͼ��������������ȷ����

(��֪��2C1O2+H2O![]() HC1O2+H++C1O3-)
HC1O2+H++C1O3-)
A. Ka(HClO2)��10-4.5
B. ������pH��ֽ�����Һ��pH
C. ���ԣ�HClO2<HClO3�������ԣ�HClO2>HClO3
D. ��ͼ�����κ�һ�㣬����c��C1O2-��+c��HC1O2��+c��C1O3-��=0.1mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ��Ҫ���뻹ԭ�����ܷ������ǣ� ��
A.Cl��Cl2B.Fe3+��Fe2+C.SO32��SO42-D.CO32��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȥ����̼���Ʒ�ĩ�е�����̼�����ƣ�������ķ����ǣ�������
A.����
B.��������������Һ
C.��������
D.����CaCl2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ִӺ�����ܷ���[������CoO��Co2O3(ֻ�����ᣬ�����ڼ�)��LiCoO2]�л��������ܵĹ�����������:

�ش���������:
(1)����I�����ܡ�ʱ��Ϊ�������ȥ���ʣ��������¶ȺͲ��Ͻ�����,���ɲ�ȡ�Ĵ�ʩ��____(�о�1��)�������ܡ�ʱ������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_______������II�����ˡ�ϴ�ӡ�����������������������Al(OH)3������Ϊ________(�����ӷ���ʽ)��
(2)����III�����ܡ�ʱ,Co2O3 ת��ΪCoSO4 �����ӷ���ʽΪ_________��
(3)����V��������ﮡ�ʱ,����pH�����÷�Χ��_______(��֪��������,Al3+��ʼ����ʱ��pHΪ4.1,������ȫʱ��pHΪ4.7.Co2+��ʼ����ʱ��pHΪ6.9��������ȫʱ��pHΪ9.4)������VI���������ijɷ�Ϊ__________��
(4)����CoC2O4ʱ����������ԭ��Ӧ����CoC2O4�ֽ�Ļ�ѧ����ʽ��___�������ӳ���ת���Ƕȿ��ǣ��ܷ����÷�ӦCoCO3+ C2O42-=== CoC2O4 + CO32- ��CoCO3 ת��ΪCoC2O4?___ (��ܡ����ܡ�),˵������:________[��֪Ksp(CoCO3) =1.4��10-13,Ksp(CoC2O4)=6.3��10-8]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ï��[(C5H5)2Fe](�Ȼ�ɫ��ĩ��������ˮ�����������ѵ��л��ܼ�)�����������ڹ�ҵ��ũҵ��ҽҩ�����졢���ܡ���������ҵ���й㷺��Ӧ�á�ijУ����С�����ݷ�Ӧ2KOH+2C5H6+FeCl2=(C5H5)2Fe+2KCl+2H2O�ھ�����ˮ���������������Ʊ���ï����
�ش���������:
(1)����ͬѧ���Ʊ���ˮFeCl2,��Ҫʵ������Ϊ:

����I�ñ���Na2CO3��Һ���ݵ�Ŀ����_______������II ����м�ǹ�����,��Ŀ����_____������IV��ˮ�ķ�����____________��
(2)�����û����ϩ������(�е�Ϊ174��)�Ʊ������ϩ(�е�Ϊ419��),��֪:(C5H6)2(�����ϩ������)![]() 2C5H6(�����ϩ)������õ������ϩ�IJ�������Ϊ________��
2C5H6(�����ϩ)������õ������ϩ�IJ�������Ϊ________��
(3)����ͬѧ�Ʊ���ï����װ����ͼ��ʾ(�г�װ������ȥ)��

��֪:���������ĽṹʽΪ �����ȶ��Ժã����ܽ������л��
�����ȶ��Ժã����ܽ������л��
��ͼ����ȴˮ�ӽӿ�______����(�a����b��)��
��װҩƷǰ������������ͨ������A��װҩƷǰͨ��N2��Ŀ����_________��
�۷�Ӧ�������ϲ�Ȼ�ɫ������Һ����������ϴ�ӣ���Ŀ����_______������ˮϴ��ˮϴʱ��˵����ϴ�Ӹɾ���������_________��ϴ�Ӻ�õ��Ķ�ï��������Һ��ö�ï������ɲ��õIJ���������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Al2(SO4)3��MgSO4�Ļ����Һ�У��μ�NaOH��Һ�����ɳ������������NaOH��Һ�������ϵ����ͼ��ʾ����ԭ���Һ��Al2(SO4)3��MgSO4�����ʵ���Ũ��֮��Ϊ( )

A. 6��1 B. 3��1 C. 2��1 D. 1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C��Ti�ĵ��ʼ��仯�������ִ�����й㷺��;��
��1����̬��ԭ�ӵĵ����Ų�ʽΪ________��
��2��CS2�����к�������������֮��Ϊ______��NO2+��CO2�ǵȵ�������NO2+�ĵ���ʽΪ___������Ϊ_______��
��3��CH3CHO�е����CH3CH2OH��ԭ����_____��CH3CHO������̼ԭ���ӻ�����Ϊ_____��
��4�����ᱵ(BaTiO3)�����ij�־�����ͼ��ʾ��NAΪ�����ӵ�����ֵ��Ba2+��O2-��Ti4+�İ뾶�ֱ�Ϊapm��bpm��cpm��

���뱵���ӵȾ������������������______����
�ڼ��辧���е�Ti4+��Ba2+�ֱ���O2-����Ӵ�����þ�����ܶȱ���ʽΪ______g.cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ҫ�Ļ���ԭ�ϣ���ҽҩ��ұ�𡢻�����ʳƷ�����㷺ʹ�á�
I. �ô�����̼���ƹ�������500mL 0.40mol/L Na2CO3��Һ��
(1)��ȡNa2CO3�����������______________________g��
(2)������Һʱ���������²��������ղ���˳��4����_________(����ĸ)��
a. ���� b. ���� c. �ܽ� d. ҡ�� e. ת�� f. ϴ�� g. ����
(3)����˵���У���ȷ����_____________________(����ĸ)��
a. ����ʱ�����ӿ̶��ߣ��ᵼ�����Ƶ���ҺŨ��ƫС
b. ����ʱ�������ˮ�����̶��ߣ�Ҫ�õι�����
c. ת��ʱ����Һ��������ƿ�⣬Ҫ����������Һ
d. ҡ�Ⱥ�Һ����ڿ̶��ߣ�Ҫ�ټ�ˮ���̶���
II. ijʵ��С���ͬѧģ���°��Ƽ��ȡ����������£�

(1)��ҵ��������ĵ�һ���dz�ȥ����ʳ��ˮ����SO42�D��Ca2+���ӣ����μ�����Լ����������� ______________�� _______________�� (����)�� _______________��
(2)��֪�������ε��ܽ��
NaCl | NH4HCO3 | NaHCO3 | NH4Cl | |
�ܽ��(20��C��100gH2Oʱ) | 36.0 | 21.7 | 9.6 | 37.2 |
��д��װ��I�з�Ӧ�Ļ�ѧ����ʽ________________________________________��
��д��װ��II�з�����Ӧ�Ļ�ѧ����ʽ________________________________��
(3)�������п�ѭ�����õ�������__________________��
(4)�Ƴ��Ĵ�����ֻ��������NaCl��
�ټ����øô������Ƶ���Һ�к���Cl�D�ķ�����_________________________��
�ڲⶨ�ô���Ĵ��ȣ����з�������������__________(����ĸ)��
a. ��m�˴�����Ʒ�м�������CaCl2��Һ�����������ˡ�ϴ�ӡ������������Ϊb g
b. ��m�˴�����Ʒ�м�������ϡ���ᣬ�ü�ʯ��(��Ҫ�ɷ���CaO��NaOH)���ղ��������壬��ʯ������b g
c. ��m�˴�����Ʒ�м�������AgNO3��Һ�������ij��������ˡ�ϴ�ӡ������������Ϊb g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com