
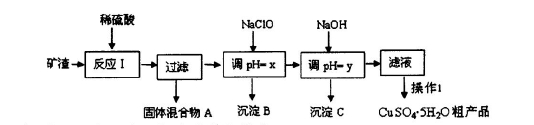
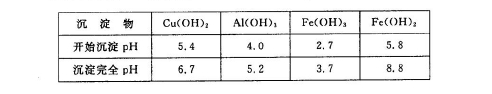
 2Cu+O2”ü+2H2SO4£®Čō¼ÓČė0£®1 molµÄCu(OH)2»į·¢Éś·“Ó¦£ŗCu(OH)2+H2SO4= CuSO4+2H2O ”£²śÉś0£®1molµÄCuSO4ŗĶ0£®2molµÄĖ®»Öø“ŌĄ“µÄČÜŅŗ”£Ōņøł¾Żµē½ā·½³ĢŹ½ÖŠµÄĪļÖŹ¼äµÄ¹ŲĻµæÉÖŖµē×Ó×ŖŅĘ0£®4mol”££Ø6£©NaClOÓŠŃõ»ÆŠŌ£¬Fe2+ÓŠ»¹ŌŠŌ”£ĖłŅŌÓĆNaClOµ÷½ŚpH£¬Éś³É³ĮµķBĪŖFe(OH)3£¬Ķ¬Ź±Éś³ÉŅ»ÖÖ¾ßÓŠĘÆ°××÷ÓƵÄĪļÖŹĪŖHClO£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe2++7ClO-+2H++5H2O=2Fe(OH)3”ż+Cl-+6HClO
2Cu+O2”ü+2H2SO4£®Čō¼ÓČė0£®1 molµÄCu(OH)2»į·¢Éś·“Ó¦£ŗCu(OH)2+H2SO4= CuSO4+2H2O ”£²śÉś0£®1molµÄCuSO4ŗĶ0£®2molµÄĖ®»Öø“ŌĄ“µÄČÜŅŗ”£Ōņøł¾Żµē½ā·½³ĢŹ½ÖŠµÄĪļÖŹ¼äµÄ¹ŲĻµæÉÖŖµē×Ó×ŖŅĘ0£®4mol”££Ø6£©NaClOÓŠŃõ»ÆŠŌ£¬Fe2+ÓŠ»¹ŌŠŌ”£ĖłŅŌÓĆNaClOµ÷½ŚpH£¬Éś³É³ĮµķBĪŖFe(OH)3£¬Ķ¬Ź±Éś³ÉŅ»ÖÖ¾ßÓŠĘÆ°××÷ÓƵÄĪļÖŹĪŖHClO£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe2++7ClO-+2H++5H2O=2Fe(OH)3”ż+Cl-+6HClO


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĶŗĶĢśÓėFeCl3ČÜŅŗ¹¹³ÉµÄŌµē³ŲÖŠ£ŗCu +2 Fe3+ = Cu2+ + 2Fe2+ |
| B£®ĢśÉĻ¶ĘŠæŹ±Ņõ¼«Īö³ö6.5gŠæ£¬ČÜŅŗÖŠ¼õÉŁZn2+ŹżĪŖ0.1mol |
| C£®ŌŚ·“Ó¦:4CuS + 5O2 = 2Cu2O + 4SO2ÖŠCuS¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į |
| D£®ÉśĢśÓėÅØH2SO4¼ÓČČ·“Ó¦æɲśÉśSO2ŗĶCO2ĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
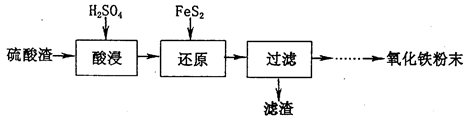
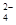 £¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________.
£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________.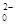
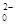 +Hg2Cl2”ż
+Hg2Cl2”ż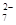 +14H+===6Fe3++2Cr3++7H2O
+14H+===6Fe3++2Cr3++7H2O| ³ĮµķĪļ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 | Mg(OH)2 | Mn(OH)2 |
| æŖŹ¼³Įµķ | 2.3 | 7.5 | 3.4 | 9.4 | 8.3 |
| ĶźČ«³Įµķ | 3.2 | 9.7 | 4.4 | 12.4 | 9.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
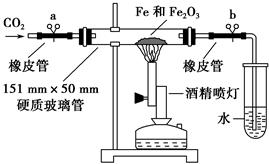
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
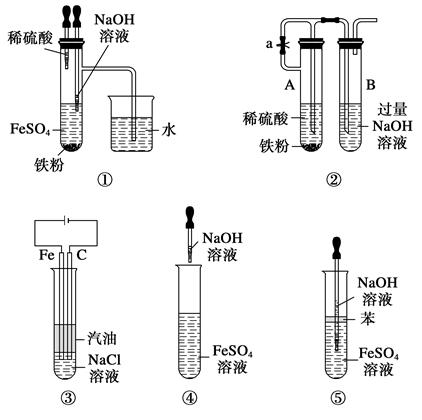
| A£®¢Ł¢Ś¢Ū¢Ü¢Ż | B£®¢Ł¢Ś¢Ū¢Ż |
| C£®¢Ł¢Ś¢Ū¢Ü | D£®¢Ś¢Ū¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
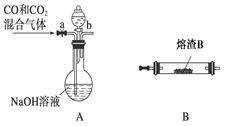

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 6SO2£«Fe3O4£¬ÓŠ3 mol FeS2²Ī¼Ó·“Ó¦£¬×ŖŅĘ________ molµē×Ó”£
6SO2£«Fe3O4£¬ÓŠ3 mol FeS2²Ī¼Ó·“Ó¦£¬×ŖŅĘ________ molµē×Ó”£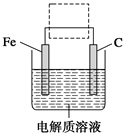
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com