
����Ŀ���������һ�����ᣬ��������ʴ��������֪25��ʱ��
HF(aq) + OH��(aq)![]() F��(aq) + H2O(l) ��H=��67.7 kJ/mol
F��(aq) + H2O(l) ��H=��67.7 kJ/mol
H+(aq) + OH��(aq)![]() H2O(l) ��H=��57.3kJ/mol
H2O(l) ��H=��57.3kJ/mol
��10 mL 0.1mol/L��NaOH��Һ�У�����10 mL Ũ��Ϊc mol/L��HFϡ��Һ������˵���д������
A. ���������Һ�¶����ߣ�HF�ĵ���̶ȼ�С�������ǻӷ���
B. ˮ������Ȼ�ѧ����ʽΪ��H2O(1)![]() H+(aq)+OH��(aq)����H= +57.3kJ/mol
H+(aq)+OH��(aq)����H= +57.3kJ/mol
C. ��c > 0.1ʱ��һ�������ڣ�c(Na+) = c(F��)
D. ����Ϻ���Һ�У�c(Na+)>c(OH��) >c(F��)>c(H+)����cһ��С��0.1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Դ������ȡ�봢��������Դ����������о��ȵ�.
��֪����CH4��g��+ H2O��g��![]() CO��g��+3H2��g�� ��H =+206.2kJ��mol-1
CO��g��+3H2��g�� ��H =+206.2kJ��mol-1
��CH4��g��+ CO2��g��![]() 2CO��g��+2H2��g�� ��H =+247.4kJ��mol-1
2CO��g��+2H2��g�� ��H =+247.4kJ��mol-1
��2H2S��g��![]() 2H2��g��+S2��g�� ��H =+169.8kJ��mol-1
2H2��g��+S2��g�� ��H =+169.8kJ��mol-1
�밴Ҫ��ش���������
(1)�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ��.CH4��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ________________.
(2)���ܱ������г���һ������H2S��������Ӧ�ۡ���ͼ��ʾΪH2S�����ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��
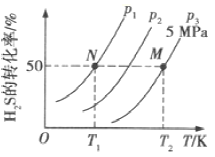
��ͼ��ѹǿ(P1��P2��P3)�Ĵ�С˳��Ϊ______���÷�Ӧƽ�ⳣ���Ĵ�С��ϵΪK��T1��_____���������������=��)K(T2)��
��������һ�����H2S��ת���ʣ����ı��¶ȡ�ѹǿ�⣬�����Բ�ȡ�Ĵ�ʩ��_________��
(3)�����Ǿ綾���壬β���������ж��ִ���������
�ټ���Һ���ա���150 ml 2.0 mol![]() L-1��NaOH��Һ����4480 mL(��״��)H2S�õ�����ҺX(�Լ���)��X��Һ������Ũ�ȵĴ�С��ϵ��ȷ����_____(��ѡ����ĸ)��
L-1��NaOH��Һ����4480 mL(��״��)H2S�õ�����ҺX(�Լ���)��X��Һ������Ũ�ȵĴ�С��ϵ��ȷ����_____(��ѡ����ĸ)��
A.c����c��Na+����c��HS-����c��S2-����c��OH-����c��H+��
B.c��Na+��+c��H+��=c��OH-��+ c��HS-��+ c��S2-��
C.2c��Na+��=3[c��H2S��+c��HS-��+ c��S2-��]
D.c��OH-��= c��H+��+ c��HS-��+2 c��H2S��
�ڴ�����Һ���գ�д�������շ�������Ӧ�����ӷ���ʽ_________________________����֪H2CO3��H2S��25��ʱ�ĵ��볣�������ʾ��

������ͭ��Һ���ա�200mL0.05 mol/ L��CuSO4��Һ����Һ��H2S��ǡ��ʹ��Ӧ��Һ��Cu2+��S2-Ũ����ȵ���Һ��c(Cu2+)Ϊ___________________(��֪�����£�Ksp(CuS)��1.0��10-36)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��������ʵ����ۻ�ԭ����һ�µ���
ѡ�� | ʵ������ | ʵ����ۻ�ԭ�� |
A | ����������ĭ�������� | 3HCO3-+Al3+===Al(OH)3��+3CO2�� |
B | ��AgCl����Һ�е�������KI��Һ���л�ɫ�������� | ˵��KSP��AgCl����KSP��AgI�� |
C | ��NaHS��Һ�е����̪����Һ���ɫ | HS-ˮ��̶ȴ��ڵ���̶� |
D | Na2CO3��Һ�еμӷ�̪�ʺ�ɫ | CO32-+2H2O |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017������й�����֪ʶ��Ȩ��ȫ������ú���Ҵ���ҵ����ĿͶ���ɹ���ij��ú���Ҵ��Ĺ��̱�ʾ���¡�
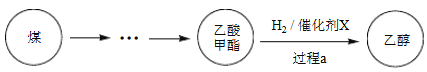
��1��Cu(NO3)2���Ʊ�������X������Ҫ�Լ���

�� ����A��_______��
�� ʵ������Cu(NO3)2����������Һ������������ϡHNO3�����û�ѧƽ��ԭ������HNO3������_______��
�� NaClO��Һ��������A�����ӷ���ʽ��_______��
��2������a��������3����Ҫ��Ӧ��
��CH3COOCH3(g)��2H2(g)![]() C2H5OH(g)��CH3OH(g) ��H1
C2H5OH(g)��CH3OH(g) ��H1
��CH3COOCH3(g)��C2H5OH(g)![]() CH3COOC2H5 (g)��CH3OH(g) ��H2
CH3COOC2H5 (g)��CH3OH(g) ��H2
��CH3COOCH3(g)��H2(g)![]() CH3CHO(g)��CH3OH(g) ��H3
CH3CHO(g)��CH3OH(g) ��H3
��ͬʱ���ڣ����CH3COOCH3ת���ʡ��Ҵ�������������ѡ���ԣ����Ҵ�ѡ����= ![]() ������ͼ��ʾ��
������ͼ��ʾ��
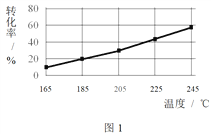
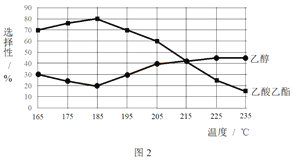
�� ��֪����H1 < 0�����¶Ƚ��ͣ���Ӧ��ѧƽ�ⳣ���ı仯������_______��
�� ����˵������������________��
A���¶ȿ�Ӱ�췴Ӧ��ѡ����
B��225�桫235�棬��Ӧ����ƽ��״̬
C������H2��Ũ�ȣ��������CH3COOCH3��ת����
�� Ϊ��ֹ����Ӧ��������Ӧ�¶�Ӧ���Ƶķ�Χ��_______��
�� ��185���£�CH3COOCH3��ʼ���ʵ���Ϊ5 mol�������Ҵ������ʵ�����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2�㷺����ҽҩ�����Ṥҵ�������շ����е�SO2�������·�����
������ | �ü�ʽ������Al2(SO4)x(OH)y��Һ���ո���SO2 |
������ | ��Fe2+��Fe3+���£��ÿ���(O2)��SO2����ΪH2SO4 |
��1��������Ĺ������¡�
�� �Ʊ�Al2(SO4)x(OH)y
��Al2(SO4)3��Һ�м���CaO��ĩ����pH��3.6�� CaO������֮һ�Ǵٽ�_______ˮ�⣨�����ӷ��ţ���ͬ�������dz���һ����_______��
�� ���գ�Al2(SO4)x(OH)y����SO2��IJ�����_______��д��ѧʽ����
�� ���������Ȣ��в������SO2��Al2(SO4)x(OH)y������
��2���������У���Fe2+���£�SO2��O2��H2O����H2SO4�Ļ�ѧ����ʽ��______��
��3���������У�Fe2+�Ĵ����̿ɱ�ʾ���£�
����2 Fe2++ SO2+ O2=2 Fe3++ SO42-
���� ����
�� д���������ӷ���ʽ��______��
�� ����ʵ�鷽����֤ʵ���������̡���ʵ�鷽������������
a����FeCl2��Һ����KSCN���ޱ仯
b����FeCl2��Һͨ������SO2������KSCN����ɫ��졣
c��ȡb����Һ��_______��
��4���������У����������õζ����ⶨ�����в���SO2�ĺ�������V L���ѻ���Ϊ��״���������е�SO2��1%��H2O2��ȫ���գ�����Һ����ͼ��ʾװ�õζ���������a mL c mol/L NaOH��Һ��
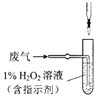
�� H2O2����SO2�Ļ�ѧ����ʽ_______��
�� �����в���SO2���������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС����Ʋ�ͬʵ�鷽���Ƚ�Cu2+��Ag+ �������ԡ�
��1������1��ͨ���û���Ӧ�Ƚ�
���ữ��AgNO3��Һ����ͭ˿��������ɫ���壬��Һ��������Ӧ�����ӷ���ʽ��_______��˵��������Ag+��Cu2+��
��2������2��ͨ��Cu2+��Ag+ �ֱ���ͬһ���ʷ�Ӧ���бȽ�
ʵ�� | �Լ� | ��ż����� | |
�Թ� | �ι� | ||
| 1.0 mol/L KI��Һ | 1.0 mol/L AgNO3��Һ | ������ɫ��������Һ��ɫ |
1.0 mol/L CuSO4��Һ | ������ɫ����A����Һ��� | ||
�� �����飬������Һ����I2����ɫ������________��
�� �����飬������Һ��I2���Ʋ�Cu2+������������ɫ����A��CuI��ȷ��A��ʵ�����£�
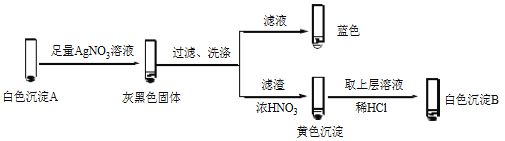
a��������Һ��I2����Һ����ɫ˵����Һ����________�������ӷ��ţ���
b����ɫ����B��________��
c����ɫ����A��AgNO3��Һ��Ӧ�����ӷ���ʽ��____��˵��������Ag+��Cu2+��
��3����������2��Ag+ δ������I- ����Cu2+������I-��ԭ�����ʵ�����£�
��� | ʵ��1 | ʵ��2 | ʵ��3 |
ʵ�� |
|
|
|
���� | �����Ա仯 | a����Һ�Ͽ���ػ�ɫ,b�е缫 ����������������ָ��ƫת | c����Һ������dz��ɫ�� ������ָ��ƫת |
���缫��Ϊʯī����ҺŨ�Ⱦ�Ϊ 1 mol/L��b��d����ҺpH��4��
�� a����Һ���ػ�ɫ��ԭ����_______���õ缫��Ӧʽ��ʾ����
�� ��ʵ��3������˵��Cu2+������I-�������ǿ����е�����Ҳ���������ã����ʵ��֤ʵ�˸����ݣ�ʵ�鷽����������_______��
�� ����2�У�Cu2+������I-,��Ag+δ������I-��ԭ��_______��
�����ϣ�Ag+ + I- = AgI�� K1 =1.2��1016��2Ag+ + 2I- = 2Ag��+ I2 K2 = 8.7��108��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�ͼ���ܽϳ�ʱ�俴��������������ɫ�������ǣ� ��


A. �٢ڢۢܢ�B. �٢ڢ�C. �٢ڢۢ�D. �ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ͨ�뵽�Ȼ�����Һ��δ������������������Һ��Ϊ���ݣ�����һ��a����NaOH��Һ����һ��b��ͨ��Cl2 �� ���а�ɫ��������������a�г����Ļ�ѧʽΪ �� b�г����Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��A��B����װ���е��ձ��ֱ�ʢ��������CuCl2��Һ��

��1��A��B��C����װ��������ԭ��ص���________(���ţ���ͬ)�����ڵ��ص���_____________
��2��A����Zn��_____�����缫��ӦʽΪ________��Cu��______�����缫��ӦʽΪ______________��A���ܷ�Ӧ�ķ���ʽΪ________________________��
��3��B�����ܷ�Ӧ�ķ���ʽΪ________________��
��4��C����Zn��____�����缫��ӦʽΪ_____��Cu��______�����缫��ӦʽΪ____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com