
| A£® | NaÓėĖ®·“Ó¦£ŗNa+2H2OØTNa++2OH-+H2”ü | |
| B£® | 0.01mol/L NH4Al£ØSO4£©2ČÜŅŗÓė0.02mol/L Ba£ØOH£©2ČÜŅŗµČĢå»ż»ģŗĻ NH4++Al3++2SO42-+2Ba2++4 OH-ØT2 Ba SO4”ż+Al£ØOH£©3”ż+NH3•H2O | |
| C£® | ¹čĖįÄĘČÜŅŗÓė“×ĖįČÜŅŗ»ģŗĻ£ŗSiO32-+2H+ØTH2SiO3”ż | |
| D£® | ÅØĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū²¢¼ÓČČ£ŗFe+3NO3-+6H+ $\frac{\underline{\;\;”÷\;\;}}{\;}$Fe3++3NO2”ü+3H2O |
·ÖĪö A£®µēŗɲ»ŹŲŗć£»
B£®ĪļÖŹµÄĮæ±ČĪŖ1£ŗ2£¬·“Ӧɜ³ÉĮņĖį±µ”¢ĒāŃõ»ÆĀĮŗĶŅ»Ė®ŗĻ°±£»
C£®·“Ӧɜ³É¹čĖįŗĶ“×ĖįÄĘ£¬“×ĖįŌŚĄė×Ó·“Ó¦ÖŠ±£Įō»ÆѧŹ½£»
D£®Fe¹żĮæ£¬Éś³ÉĻõĖįŃĒĢś”¢¶žŃõ»ÆµŖŗĶĖ®£®
½ā“š ½ā£ŗA£®NaÓėĖ®·“Ó¦µÄĄė×Ó·“Ó¦ĪŖ2Na+2H2OØT2Na++2OH-+H2”ü£¬¹ŹA“ķĪó£»
B.0.01mol/L NH4Al£ØSO4£©2ČÜŅŗÓė0.02mol/L Ba£ØOH£©2ČÜŅŗµČĢå»ż»ģŗĻµÄĄė×Ó·“Ó¦ĪŖNH4++Al3++2SO42-+2Ba2++4OH-ØT2BaSO4”ż+Al£ØOH£©3”ż+NH3•H2O£¬¹ŹBÕżČ·£»
C£®¹čĖįÄĘČÜŅŗÓė“×ĖįČÜŅŗ»ģŗĻµÄĄė×Ó·“Ó¦ĪŖSiO32-+2CH3COOHØTH2SiO3”ż+22CH3COO-£¬¹ŹC“ķĪó£»
D£®ÅØĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū²¢¼ÓČȵĥė×Ó·“Ó¦ĪŖFe+2NO3-+4H+ $\frac{\underline{\;\;”÷\;\;}}{\;}$Fe2++2NO2”ü+2H2O£¬¹ŹD“ķĪó£»
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éĄė×Ó·“Ó¦·½³ĢŹ½ŹéŠ“µÄÕżĪóÅŠ¶Ļ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĻ°·¢ÉśµÄ·“Ó¦¼°Ąė×Ó·“Ó¦µÄŹéŠ“·½·ØĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲŃõ»Æ»¹Ō·“Ó¦”¢ø“·Ö½ā·“Ó¦µÄĄė×Ó·“Ӧ漲飬עŅāĄė×Ó·“Ó¦ÖŠ±£Įō»ÆѧŹ½µÄĪļÖŹ¼°µē×Ó”¢µēŗÉŹŲŗć£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| µēĄė³£Źż Ėį | K1 | K2 |
| H2SO3 | 1.3”Į10-2 | 6.3”Į10-8 |
| H2CO3 | 4.2”Į10-7 | 5.6”Į10-11 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±½ŌŚ³£ĪĀĻĀæÉÓėäåĖ®·¢ÉśČ”“ś·“Ó¦ | |
| B£® | ĘĻĢŃĢĒÄÜ·¢ÉśŃõ»Æ·“Ó¦ŗĶŅų¾µ·“Ó¦ | |
| C£® | ŅŅ“¼ŗĶŅŅĖį¶¼ÄÜÓėĒāŃõ»ÆÄĘ·“Ó¦ | |
| D£® | ĢĒĄą”¢ÓĶÖ¬ŗĶµ°°×ÖŹ¶¼ÄÜ·¢ÉśĖ®½ā·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijŠĖȤŠ”×éĶ¬Ń§ŃŠ¾æĮĖŃõ×åŌŖĖŲ¼°ĘäijŠ©»ÆŗĻĪļµÄ²æ·ÖŠŌÖŹ£¬²éŌÄ׏ĮĻČēĻĀ£ŗ
ijŠĖȤŠ”×éĶ¬Ń§ŃŠ¾æĮĖŃõ×åŌŖĖŲ¼°ĘäijŠ©»ÆŗĻĪļµÄ²æ·ÖŠŌÖŹ£¬²éŌÄ׏ĮĻČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
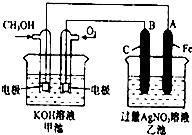 ČēĶ¼ŹĒŅ»øö»Æѧ¹ż³ĢµÄŹ¾ŅāĶ¼£®ŅŃÖŖ¼×³ŲµÄ×Ü·“Ó¦Ź½ĪŖ£ŗ2CH3OH+3O2+4KOHØT2K2CO3+6H2O ĢīŠ“ĻĀĮŠæÕ°×£ŗ
ČēĶ¼ŹĒŅ»øö»Æѧ¹ż³ĢµÄŹ¾ŅāĶ¼£®ŅŃÖŖ¼×³ŲµÄ×Ü·“Ó¦Ź½ĪŖ£ŗ2CH3OH+3O2+4KOHØT2K2CO3+6H2O ĢīŠ“ĻĀĮŠæÕ°×£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £»Na2O2ÓėSO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Na2O2+2SO2ØT2Na2SO4£®
£»Na2O2ÓėSO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Na2O2+2SO2ØT2Na2SO4£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
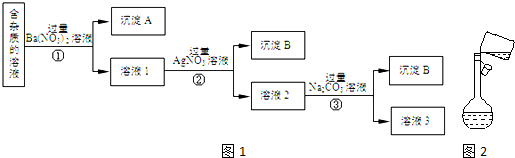
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé·½·Ø | ĻÖĻó | ½įĀŪ |
| ĻņijČÜŅŗ¼ÓČėAgNO3ČÜŅŗ | ³öĻÖ°×É«³Įµķ | ŌČÜŅŗÖŠŅ»¶ØÓŠCl- | |
| ĻņijČÜŅŗ¼ÓČėBaCl2ČÜŅŗ | ³öĻÖ°×É«³Įµķ | ŌČÜŅŗÖŠŅ»¶ØÓŠSO42- | |
| ĻņijČÜŅŗ¼ÓČėNaOHČÜŅŗ | ³öĻÖĄ¶É«³Įµķ | ŌČÜŅŗÖŠŅ»¶ØÓŠCu2+ | |
| ĻņijČÜŅŗ¼ÓČėH2SO4ČÜŅŗ | ²śÉśĪŽÉ«ĪŽĪ¶ĘųĢå | ŌČÜŅŗÖŠŅ»¶ØÓŠCO32- |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com