
 ��
��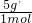 =4��5��
=4��5�� =0.3mol��HCl��������ʵ���Ϊ
=0.3mol��HCl��������ʵ���Ϊ =0.5mol��
=0.5mol�� =0.4mol��NH3�����ʵ���Ϊ0.2mol��
=0.4mol��NH3�����ʵ���Ϊ0.2mol��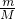 ��pM=��RT�����㣻
��pM=��RT�����㣻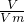 =
=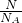 =
=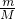 ���������ʵ�������������������ʵ��������������
���������ʵ�������������������ʵ��������������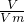 =
=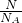 =
=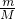 ��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ��
��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ�� 

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013����ʡ۳����һ�и�һ��һ�ο��Ի�ѧ�Ծ����������� ���ͣ������
��10�֣���1��ͬ��ͬѹ�£���������A��B�����֮��Ϊ2��1������֮��Ϊ8��5����A��B���ܶ�֮��Ϊ ��Ħ������֮��Ϊ ��
��2���ڱ�״����a. 6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ��
�ڱ�״���������������� ��
��������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�������ʮ����ѧ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
���㣨18�֣�
��1��ͬ��ͬѹ�µ�������ͬ�����зֱ�װ��O2��O3���壬����ƿ�����з�����֮��
�� ��ԭ����֮���� ������֮���� ���ܶ�֮����
��2�������������ļ��������У����з����������� ������ԭ��������
�� ����״������������� ��
�� 1gH2�� �� 2.408��1023��CH4���� 10.8gH2O�� �� ��״����6.72LCO2
��3����NA��ʾ�����ӵ����������agij���庬�еķ�������b����cg��������
��״���µ������ ���ú�NA��ʽ�ӱ�ʾ����
��4������100 mL 1mol/L�ĵ�ϡH2SO4��Һ����Ҫ����Ͳ��ȡ��������Ϊ98%��
ŨH2SO4���ܶ�Ϊ1.84g/cm3�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013����ʡ��һ��һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣���1��ͬ��ͬѹ�£���������A��B�����֮��Ϊ2��1������֮��Ϊ8��5����A��B���ܶ�֮��Ϊ ��Ħ������֮��Ϊ ��
��2���ڱ�״����a. 6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ��
�ڱ�״���������������� ��
��������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡׯ���и�һ��ѧ�ڵ�һ���¿���ѧ�� ���ͣ�ѡ����
��10�֣���1��ͬ��ͬѹ�£���������A��B�����֮��Ϊ2��1������֮��Ϊ8��5����A��B���ܶ�֮��Ϊ ��Ħ������֮��Ϊ ��
��2���ڱ�״����a. 6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ���ڱ�״���������������� ��
��������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com