
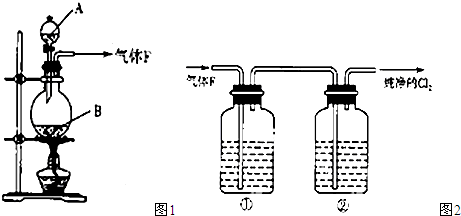
��ͼ1��ʾ��ij��ȤС��ͬѧ��ͭƬ����ϡ���ᣬ���ֿ�ʼʱ���ݲ������ʷdz�����һ��ʱ����������Լӿ죬��ƿ����Һ��dz��ɫ�����ϼ��Һ���Ϸ���������ɫҲ�ڲ��ϼ����С��ͬѧ��ͨ��ʵ��̽����Ӧ���ʱ仯��ԭ��

ͼ 1 ͼ 2
(1) ͼ1��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ ��
�����ӷ���ʽ��ʾNaOH��Һ��������� ��
(2) С��ͬѧ��������¼��貢���ʵ��̽����
��. ��ͬѧ��Ϊ�Ƿ�Ӧ���ȵ�����Һ�¶��������£���ɴ�ʵ�黹��Ҫ�������� ��
�ⶨ��Ӧ��������Һ��ͬʱ����¶ȣ�������±���
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 35 | 50 | 60 | 70 | 80 |
| �¶�/�� | 25 | 26 | 26 | 26 | 26 | 26 | 26.5 | 27 | 27 | 27 | 27 |
���ʵ��Ŀ�ĺͱ������ݣ���ó��Ľ����� ��
��. ��ͬѧ��Ϊ���ɵ�Cu2+�Է�Ӧ�д����ã�Ϊ��֤�˼��裬ȡA��B��֧�Թֱܷ���������ͭƬ��ϡ���ᣬ��ô�����������һ֧�Թ��м��������� ������ţ���
A. ����ͭ���� B. ����ͭ��Һ C. ����ͭ���� D. ����ͭ��Һ
Ȼ��Ա���֧�Թܵķ�Ӧ���������������ͬ����˵ó����ۣ�Cu2+�����Ƿ�Ӧ�Ĵ�����
��. ��ͬѧ���������ƲⷴӦ�����л������� ���ɣ�������Ϊ�����ʶԷ�Ӧ�д����ã���ͼ2��ʾ��ʵ���б�ͬѧ��a��ͨ������ʺ�������в��������������Կ����ҹܡ�С��ͬѧ�ó������ۣ��������ʶ�ͭ��ϡ����ķ�Ӧ�д����á�
��3��ʵ����������Թ�����Һ����ɫ����������ɫ������ͬѧ��Ϊ�Ǹ���Һ������ͭ�����������ϸ����£���һ����ͬѧ��Ϊ�Ǹ���Һ���ܽ���ͨ������ʡ��������һ��ʵ�鷽����֤�����ּ�����ȷ��(д��ʵ�������ʵ������ͽ���)
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 CH3COOH+OH-
CH3COOH+OH- CH3COOH+OH-
CH3COOH+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
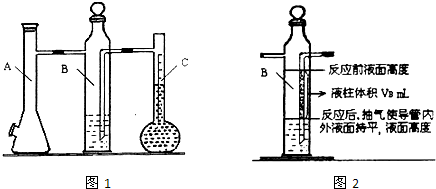
| ʵ����� | m��Mg��/g | �������/mL | Һ����ƿ��Һ�����/mL | ����������/mL | �������/mL | ����1mol�����/L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | X | |
| 2 | 0.115 | 10.0 | 121.0 | 8.0 |
| ʵ����� | m��Mg�� g |
�������mL | Һ����ƿ��Һ�����mL | ����������mL | Bƿ��һ��Һ�����mL | ˮ������ٷֺ��� | ����1mol�����L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | VB | a% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| H | + 4 |
| O | - 2 |
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com