
�м���Ԫ�ص����ĺ�����Ӳ�ṹ��ͼ��ʾ��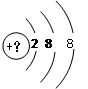
��ش��������⡣
��1�������������������������ķ�����_____________��
��2��������������Һ��ʹ��ˮ��ɫ�������ֻ��ǣ��������ķ�����____________��
��3���������������Ժ������õ�1�����Ӻ��Ϊԭ�ӣ��䵥�ʵĻ�ԭ�Ժ�ǿ���������ķ�����________________��
��4���������Ļ�ԭ�Ժ�����ʧȥ1�����Ӻ��Ϊԭ�ӣ��䵥�ʵ������Ժ�ǿ���������ķ�����_______________
��1��Ar ��2��S2�� ��3�� K�� (4) Cl��
���������������1���������������������������������������2+8+8��18������������ķ�����Ar��
��2��������������Һ��ʹ��ˮ��ɫ�������ֻ��ǣ�˵���������л�ԭ�ԣ������������ķ�����S2����
��3���������������Ժ������õ�1�����Ӻ��Ϊԭ�ӣ��䵥�ʵĻ�ԭ�Ժ�ǿ�����Ӧ���ǻ��õļ��������������2+8+8+1��19�������������ķ�����K����
��4���������Ļ�ԭ�Ժ�����ʧȥ1�����Ӻ��Ϊԭ�ӣ��䵥�ʵ������Ժ�ǿ����������������������ķ�����2+8+8��1��17�������������ķ�����Cl����
���㣺�����������Ų��������Ժͻ�ԭ�ԡ��������������������ϵ���жϵ�
���������������ӱ��������ǿ�����ض�ѧ����������������������������ѧ���������������ͷ�ɢ˼ά��������ȷ���ĺ�����������������Ĺ�ϵ�Ǵ���Ĺؼ���


 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�йض�����Ԫ��A��B��C��D��E��F����Ϣ���£�
| Ԫ�� | �й���Ϣ |
| A | ����������Ӧ��ˮ����ף���������̬�⻯��ң���Ӧ������ |
| B | �����������Ǵ�����������2�� |
| C | M������3������ |
| D | ������ԭ�Ӱ뾶��������Ԫ�� |
| E | �䵥���ǵ���ɫ���� |
| F | �������������۴�����Ϊ6 |

 �� DF�ĵ���ʽΪH��Cl��
�� DF�ĵ���ʽΪH��Cl���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֶ���������Ԫ��A��B��C�� D��E����ԭ��������������Aԭ��Լռ������ԭ��������88.6%��A+�ֳ�Ϊ���ӣ�B���γɻ�������������Ԫ�أ�CԪ�ص�����⻯��Y��ˮ��Һ�Լ��ԣ�E�Ƕ�����Ԫ���е縺����С��Ԫ�ء�A��B��C��E����Ԫ�ض�����DԪ���γ�ԭ�Ӹ����Ȳ���ͬ�ij���������Իش��������⣺
��1��д��A��E��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ____��
��2�����Ȼ�������Һ�μӹ�����E������������Ӧˮ�������Һ��������____��
��3��Y��Һ�Լ��Ե�ԭ����(��һ�����ӷ���ʽ��ʾ��____��
��4����������β���к��еĻ�����BD�ķ����ǣ�������PdC12��Һ��ͨA����β���������ɺ�ɫ������Pd����֤������β���к���BD��д����Ӧ�����ӷ���ʽ____��
��5�������й��������ʵıȽ��У�����ȷ���� ��
a�����ȶ��ԣ�H2S>SiH4 b�����Ӱ뾶��Na+>S2��
c����һ������N>O d��Ԫ�ص縺�ԣ�C>H
��6����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
W��X��Y��ZΪ���ֶ���������Ԫ�أ���λ�ù�ϵ��ͼ��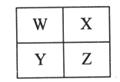
��1����Xԭ�ӵ������������Ǵ�����3����
��Ԫ��X�����ڱ��е�λ��___________________________________��
�ڹ�ҵ����W�ĵ����Ʊ�W����̬�⻯��Ļ�ѧ����ʽ��
_______________________________________________________________________��
��2��������Ԫ����ֻ��һ�ֽ���Ԫ�ء�
��ұ��Y�Ļ�ѧ����ʽ��__________________________________________��
�ڹ�ҵ�ϣ���X�ĵ�����Z�������������ȵ�1900�����Ͽ��Ƶ�һ�������մɣ�ZX����ͬʱ�õ�һ�ֿ�ȼ�����壬�÷�Ӧ�Ļ�ѧ����ʽ��
_________________________________
��Ϊ�Ƚ�X��Z����������ˮ��������ǿ����ijͬѧ�������ʵ�顣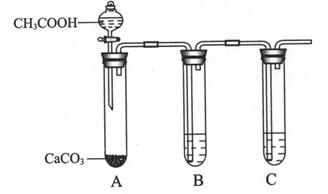
I. B�Թ���ʢ�ŵ��Լ���______________________��
II. C�Թ��з�Ӧ�Ļ�ѧ����ʽ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C�ǵڶ����ڵķǽ���Ԫ�أ���ԭ��������������������֮������γ�AC��BC�Լ�AC2��BC2���ӣ�DԪ����һ�ֶ�����Ԫ�أ�����A��B��C�ɷֱ��γɵ���������ȵ����ַ��ӡ�����գ�
(1)д��A��B��C��D��Ӧ��Ԫ�ط��ţ�A________��B________��C________��D________��
(2)д����ҵ������BD3��������Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(3)����ΪB��C��DԪ���γɵĻ�����֮��________(��ܡ����ܡ�)��������ķ�ӦBC��BD3�D��DBC2��D2C���ó�������۵�������__________________________ _��
(4)��.���º�ѹ�£���һ����ɱ���ܱ������з������з�Ӧ��4AC(g)��2BC2(g)  4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��
4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��
��. ��ά���¶Ȳ��䣬��һ�����Ӧǰ����ʼ�����ͬ���ݻ��̶����ܱ������з������������Ļ�ѧ��Ӧ����ʼʱ���������г���AC��BC2��1 mol����ƽ��ʱ����AC2��B2��b mol����b����е�a���бȽϣ���a________b(���������������������ȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҡ������������ֶ�����Ԫ�أ���ԭ����K����M���������ȣ���ԭ�ӵĺ������������ԭ�Ӻ����������1����ԭ�ӵ������������Ǵ�����������2������ԭ�Ӻ˵�����ȱ�ԭ�Ӻ˵������2����ش�
��1���ĵ�����ˮ��Ӧ�����ӷ���ʽΪ ��
��2����Ԫ�������ڱ��е�λ��Ϊ ��
��3����Ԫ�ص����������ĵ���ʽΪ ��
��4�����붡����Ԫ�ؿ���ɵľ���ǿ�����Ե������� �����ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣�����X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�ء�Xԭ�ӵ������������Ǻ�����Ӳ�����3����Y���������������۵Ĵ�����Ϊ6��X��Zͬ���壬W��X��Y����ͬ���ڣ�R2+�ļ۵����Ų�ʽΪ3d5��
��1��RԪ�������ڱ��е�λ�� ��W2X2�ĵ���ʽ ��
��2��X��Y��Z����ͼ��⻯���У��е���ߵ��� ���ѧʽ�������ۼ�������С���� ��X��Y��Z���ӻ����� ������ͬ����ͬ����
��3����R�ľ�����ÿ������ƽ������2��Rԭ�ӣ�������ͼ��������?���δ������Rԭ�ӡ�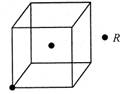
��4����1.19g ZXY2����100mlˮ�з�����������ԭ��Ӧ������2���ᣬ��Ӧ����ʽΪ ��������Һ��������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣� A��B��C��D��E��F��G����Ԫ�أ����������������ƶϣ���A��B��C��ͬһ���ڵĽ���Ԫ�أ�ԭ�Ӻ������������Ӳ㣬A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶A>B>C����D��E�Ƿǽ���Ԫ�أ����Ǹ��������Ͽ�������̬�⻯��HD��HE������ʱ��D�ĵ�����Һ�壬E�ĵ����ǹ���
��F�ڳ����������壬�����ȶ����dz��������������̬��G�dz�����ԭ�Ӱ뾶��С��Ԫ��
��1��A �������� ��Bλ�����ڱ��е� ���ڵ� �壬C��ԭ�ӽṹʾ��ͼ��
��2��AԪ����DԪ���γɻ�����ĵ���ʽ��
��3��F��Ԫ�ط�����
��4������������Ԫ���У�����������Ӧ��ˮ���������ǿ�Ļ�ѧʽ�� ��������ǿ�Ļ�ѧʽ�� ����̬�⻯�����ȶ��Ļ�ѧʽ��
��5����C���������Ӧ��ˮ����Ͷ�뵽A���������Ӧ��ˮ�����У���Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1����������������,�뾶���ɴ�С������˳������������������
�ٻ�̬X��ԭ�ӽṹʾ��ͼ:��+16
�ڻ�̬Y�ļ۵����Ų�ʽ:3s23p5
�ۻ�̬Z2-�ĵ����Ų�ͼ:
��W��̬ԭ����2���ܲ�,����ʽ:�á�
��2����֪An+��B(n+1)+��Cn-��D(n+1)-��������ͬ�ĵ��Ӳ�ṹ,��A��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳������������������,���Ӱ뾶�ɴ�С��˳��������������������,ԭ�������ɴ�С��˳��������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com