
��10�֣��Ӻ�����п����Ͳ��Ľ���ͭ��������ȡ����������һ�ֹ������£��߿���Դ��
����ˮ��Ũ������Ũ����Ļ����߿���Դ��
 �߿���Դ��
�߿���Դ��
�߿���
�������Ϲ��ջش��������⣺
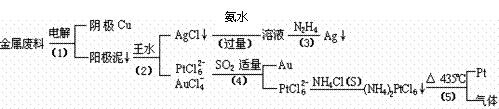
��1�����ʱ����ͭ��������Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ��д�������缫��Ӧ����ʽ�� ��
��2���£�N2H4���ǻ�����䳣�õ�һ��Һ��ȼ�ϣ�����ȫȼ��ʱ������������Ⱦ�����塣��֪���ֹ��ۼ��ļ����������±���
��ѧ�� | N-N | O=O | N��N | O-H | N-H |
����/kJ?mol-1 | 193 | 499 | 941 | 460 | 393 |
������ȫȼ��ʱ���Ȼ�ѧ����ʽΪ�� ��
��3��д�����裨4�������ӷ�Ӧ����ʽ�� ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au + 6HNO3��Ũ��= Au(NO3)3 + 3NO2��+ 3H2O�����÷�Ӧ��ƽ�ⳣ����С�����Խ��Ũ���Ἰ������Ӧ������ȴ����������ˮ���Լ�Ҫ����ԭ�� ��
��5����֪������(NH4)2PtCl6��KspԼΪ9��10��6�����裨4��������Һ��PtCl62��Ũ��Ϊ1��10��4mol?L- 1����Ҫ��1 m3�ĸ���Һ�п�ʼ����(NH4)2PtCl6�������������NH4Cl���������Ϊ kg��������Һ����ı仯���Բ��ƣ�
��1��Zn�D2e�D ��Zn2+��Cu�D2e�D ��Cu2+��2�֣�
��2��N2H4(l)+O2(g)=N2(g)+2H2O(g)����H= -517 kJ?mol1��2�֣�
��3��2AuCl4�D + 3SO2 + 6 H2O = 2Au + 8Cl�D + 3SO42�D + 12H+ ��2�֣�
��4����ˮ�к��д�����Cl�D��Au3+��Cl�D������AuCl4�D��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ��������Ӧ�����ƶ�����������ˮ����2�֣�
��5��16.05��2�֣�


 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com