
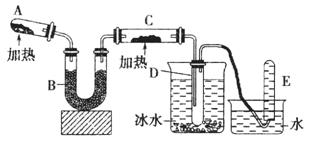
ŃĒĀČĖįÄĘ(NaClO2)ŹĒŅ»ÖÖÖŲŅŖµÄĻū¶¾¼Į”£ŅŃÖŖ£ŗ¢ŁNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ŹŹµ±Ģõ¼žĻĀæɽį¾§Īö³öNaClO2”¤3H2O£¬¢ŚClO2µÄ·ŠµćĪŖ283K£¬“æClO2Ņ×·Ö½ā±¬ÕØ£¬¢ŪHClO2ŌŚ25”ꏱµÄµēĄė³Ģ¶ČÓėĮņĖįµÄµŚ¶ž²½µēĄė³Ģ¶ČĻąµ±£¬æÉŹÓĪŖĒæĖį”£ČēĶ¼ŹĒ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘµÄ¹¤ŅÕĮ÷³ĢĶ¼£ŗ
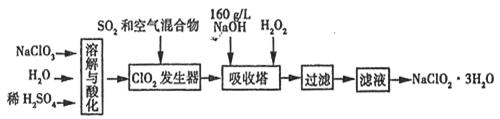
(1)C1O2·¢ÉśĘ÷ÖŠĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆæÉÄÜŹĒ (Ń”ĢīŠņŗÅ)”£
A£®½«SO2Ńõ»Æ³ÉSO3ŌöĒæĖįŠŌ B£®Ļ”ŹĶC1O2ŅŌ·ĄÖ¹±¬ÕØ
C£®½«NaClO3Ńõ»Æ³ÉC1O2
(2)ŌŚøĆŹµŃéÖŠÓĆÖŹĮæÅØ¶ČĄ“±ķŹ¾NaOHČÜŅŗµÄ×é³É£¬ČōŹµŃ鏱ŠčŅŖ450ml
l60g£ÆLµÄNaOHČÜŅŗ£¬ŌņŌŚ¾«Č·ÅäÖĘŹ±£¬ŠčŅŖ³ĘČ”NaOHµÄÖŹĮæŹĒ g£¬
ĖłŹ¹ÓƵÄŅĒĘ÷³żĶŠÅĢĢģĘ½”¢ĮæĶ²”¢ÉÕ±”¢²£Į§°ōĶā£¬»¹±ŲŠėÓŠ
(3) ĪüŹÕĖžÄŚµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬ĘäÖ÷ŅŖÄæµÄŹĒ _£¬ĪüŹÕĖžÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
(4)ŌŚĪüŹÕĖžÖŠ£¬æÉ“śĢęH2O2µÄŹŌ¼ĮŹĒ (ĢīŠņŗÅ)”£
A£®Na2O2 B£®Na2S C£®FeCl2 D£®KMnO4
(5)“ÓĀĖŅŗÖŠµĆµ½NaClO2”¤3H2O¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“ĪŹĒ £ØĢī²Ł×÷Ćū³Ę£©
A£®ÕōĮó B£®Õō·¢ÅØĖõ C£®×ĘÉÕ D£®¹żĀĖ E”¢ĄäČ“½į¾§
”¾ÖŖŹ¶µć”湤ŅÕĮ÷³ĢĢāA1 D2 D3 J1 J2
”¾“š°ø½āĪö”æ
£Ø1£©2ClO3”Ŗ+SO2=2ClO2+SO42”Ŗ B £Ø2£©80.0 500mlČŻĮæĘæ,½ŗĶ·µĪ¹Ü
£Ø3£©ĪĀ¶ČÉżøߣ¬H2O2Ņ×·Ö½ā 2ClO2+H2O2 +2NaOH=2NaClO2+2H2O+O2
£Ø4£©A £Ø5£©BED»ņED(2·Ö)
(·½³ĢŹ½¼°(5) 2·Ö£¬ĘäÓąĆææÕ1·Ö)
½āĪö£ŗ£Ø1£©·ÖĪöĮ÷³ĢĶ¼ÖŖClO3-”śC1O2£¬ClŌŖĖŲµÄ»ÆŗĻ¼Ū½µµĶ£¬¼“·¢ÉśĘ÷ÖŠĖł·¢Éś·“Ó¦ŹĒClO3-Ńõ»Æ¶žŃõ»ÆĮņ£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖSO2+2ClO3-=2C1O2+SO42-£»øł¾Ż“æClO2Ņ×·Ö½ā±¬ÕØÖŖ·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆÓ¦ŹĒĻ”ŹĶClO2£¬ŅŌ·ĄÖ¹±¬ÕØ”£
£Ø2£© £Ø3£©øł¾ŻClO2”śNaClO2ÖŖĪüŹÕĖžÄŚµÄ·“Ó¦ŹĒClO2Ńõ»ÆH2O2£¬¼“2ClO2+H2O2 +2NaOH=2NaClO2+2H2O+O2£¬¶ųĪĀ¶ČøßH2O2»į·Ö½ā”£
£Ø4£©ŌŚĪüŹÕĖžÖŠ£¬æÉ“śĢęH2O2µÄŹŌ¼ĮŹĒ»¹ŌŠŌÓėĘä½Ó½üµÄ£¬æÉŃ”Na2O2£¬²»ÄÜŃ”ÓĆ»¹ŌŠŌĢ«ĒæµÄNa2S”¢FeCl2£¬·ńŌņµĆ²»µ½NaClO2£¬¶ųĒŅÓĆFeCl2æÉÄÜŅżČėŌÓÖŹ”£
£Ø5£©ÓÉÓŚNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬Ņņ“ĖæÉĶعżĄäČ“½į¾§”¢¹żĀĖµĆµ½¾§Ģ壬ČōČÜŅŗÅضČĘ«Š”£¬æÉĻČÕō·¢ÅØĖõ£¬ŗóĄäČ“½į¾§”¢¹żĀĖ”£
”¾Ė¼Ā·µć²¦”æ׊ĻøÉóĢā»ńČ”ŠÅĻ¢ŹĒ½āĢāµÄ¹Ų¼ü£¬Čē±¾ĢāµÄ”°NaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó”±”¢”°“æClO2Ņ×·Ö½ā±¬ÕØ”±”¢”°C1O2·¢ÉśĘ÷”±”¢Ņžŗ¬ŠÅĻ¢Čē”°ĪĀ¶ČøßH2O2»į·Ö½ā”±”¢·“Ó¦Ē°ŗóĪļÖŹĖłŗ¬ŌŖĖŲµÄ»ÆŗĻ¼ŪµČ”£


 øßÖŠ±ŲĖ¢ĢāĻµĮŠ“š°ø
øßÖŠ±ŲĖ¢ĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌĻĀĮŠø÷ČÜŅŗÖŠ£¬Ī¢Į£µÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµ±ķŹöÕżČ·µÄŹĒ
A.0.1mol·L-1µÄ(NH4)2CO3ČÜŅŗÖŠ:c(CO32-)>c(NH4+)>c(H+)>c(OH-)
B. 0.1 mol·L-1µÄNaHCO3ČÜŅŗÖŠ:c(Na£«)=c(HCO3£)+c(H2CO3)+2c(CO32£)
C.½«0.2 mol·L-1 NaAČÜŅŗŗĶ0.1 mol·L-1ŃĪĖįµČĢå»ż»ģŗĻĖłµĆ¼īŠŌČÜŅŗÖŠ:c(Na£«)+c(H+)=c(A-)+c(Cl-)
D.ŌŚ25”ꏱ£¬1mol·L-1µÄCH3COONaČÜŅŗÖŠ:c(OH-)=c(H+)+c(CH3COOH)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲŹµŃé×°ÖĆ½ųŠŠµÄĻąÓ¦ŹµŃ飬²»ÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ £Ø £©
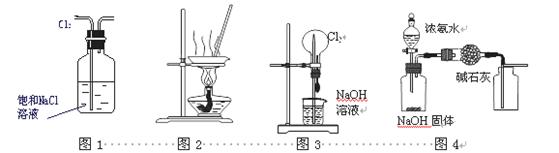
A£®ÓĆĶ¼1ĖłŹ¾×°ÖĆ³żČ„Cl2ÖŠŗ¬ÓŠµÄÉŁĮæHCl
B£®ÓĆĶ¼2ĖłŹ¾×°ÖĆÕō·¢KClČÜŅŗÖʱøĪŽĖ®KCl
C£®ÓĆĶ¼3ĖłŹ¾×°ÖĆæÉŅŌĶź³É”°ÅēČŖ”±ŹµŃé
D£®ÓĆĶ¼4ĖłŹ¾×°ÖĆÖĘČ”øÉŌļ“æ¾»µÄNH3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĮĖÄ£Äā¹¤ŅµĮ÷³Ģ“ÓÅØĖõµÄŗ£Ė®ÖŠĢįČ”Ņŗä壬²éŌÄ׏ĮĻÖŖ£ŗBr2µÄ·ŠµćĪŖ59”ę£¬Ī¢ČÜÓŚĖ®£¬ÓŠ¶¾ŠŌ”£Éč¼ĘĮĖČēĻĀ²Ł×÷²½Öč¼°Ö÷ŅŖŹµŃé×°ÖĆ£Ø¼Š³Ö×°ÖĆĀŌČ„£©£ŗ
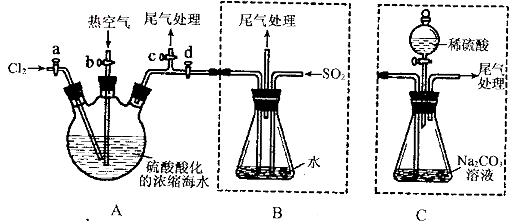
¢ŁĮ¬½ÓAÓėB£¬¹Ų±Õ»īČūb”¢d£¬“ņæŖ»īČūa”¢c£¬ĻņAÖŠ»ŗĀżĶØČėÖĮ·“Ó¦½įŹų£»
¢Ś¹Ų±Õa”¢c£¬“ņæŖb”¢d£¬ĻņAÖŠ¹ÄČė×ćĮæČČæÕĘų£»
¢Ū½ųŠŠ²½Öč¢ŚµÄĶ¬Ź±£¬ĻņBÖŠĶØČė×ćĮæSO2:
¢Ü¹Ų±Õb£¬“ņæŖa£¬ŌŁĶعżAĻņBÖŠ»ŗĀżĶØČė×ćĮæCl2;
¢Ż½«BÖŠĖłµĆŅŗĢå½ųŠŠÕōĮó£¬ŹÕ¼ÆŅŗä唣
Ēė»Ų“š£ŗ
(1)ŹµŃéŹŅÖŠ³£ÓĆĄ“ÖʱøĀČĘųµÄ»Æѧ·½³ĢŹ½ĪŖ__________________________£»
(2)²½Öč¢ŚÖŠ¹ÄČėČČæÕĘųµÄ×÷ÓĆĪŖ_____________________________£»
(3)²½ÖčBÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________”£
(4)“ĖŹµŃéÖŠĪ²ĘųæÉÓĆ £ØĢīŃ”Ļī×ÖÄø£©ĪüŹÕ“¦Ąķ”£
a£®Ė® b£®ÅØĮņĖį c£®NaOHČÜŅŗ d.±„ŗĶNaCIČÜŅŗ
(5)²½Öč¢ŻÖŠ£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÕōĮó£¬ŹÕ¼ÆŅŗä壬½«×°ÖĆĶ¼ÖŠČ±ÉŁµÄ±ŲŅŖŅĒĘ÷²¹»³öĄ“”£
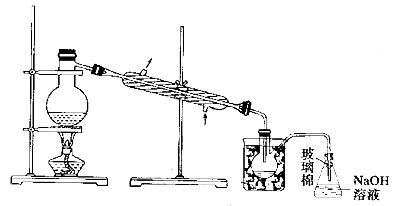
(6)ČōÖ±½ÓĮ¬½ÓAÓėC£¬½ųŠŠ²½Öč¢ŁŗĶ¢Ś£¬³ä·Ö·“Ó¦ŗó£¬Ļņ׶ŠĪĘæÖŠµĪ¼ÓĻ”ĮņĖį£¬ŌŁ¾²½Öč¢Ż£¬Ņ²ÄÜÖʵĆŅŗä唣µĪ¼ÓĻ”ĮņĖįÖ®Ē°£¬CÖŠ·“Ӧɜ³ÉĮĖNaBrO3µČ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________”£
(7)ÓėB×°ÖĆĻą±Č£¬²ÉÓĆC×°ÖƵÄÓŵćĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ°±æÉŅŌÓė×ĘČȵÄŃõ»ÆĶ·“Ó¦µĆµ½µŖĘųŗĶ½šŹōĶ£¬ÓĆŹ¾ŅāĶ¼ÖŠµÄ×°ÖĆæÉŅŌŹµĻÖøĆ·“Ó¦”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AÖŠÉś³É°±Ęų·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_________________________________________£»
(2)BÖŠ¼ÓČėµÄøÉŌļ¼ĮŹĒ_________(ĢīŠņŗÅ)¢ŁÅØĮņĖį¢ŚĪŽĖ®ĀČ»ÆøĘ ¢Ū¼īŹÆ»Ņ£»
(3)ÄÜÖ¤Ć÷°±ÓėŃõ»ÆĶ·“Ó¦µÄĻÖĻó¢ŁCÖŠ______________”¢¢ŚDÖŠÓŠĪŽÉ«ŅŗĢåÉś³É£»
Éč¼ĘŹµŃé¼ģŃéDÖŠĪŽÉ«ŅŗµÄ³É·Ö£ŗȔɣĮæŅŗĢåÓŚŹŌ¹ÜÖŠ£¬¼ÓČėÉŁĮæ________·ŪÄ©£¬ĻÖĻóĪŖ___________________”£
(4)Š“³ö°±ĘųÓėŃõ»ÆĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½___________________________£»ČōŹÕ¼Æµ½2.24L(STP)µŖĘų£¬¼ĘĖć×ŖŅʵē×ÓŹżĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķ¬ĖŲŅģŠĪĢåĻą»„×Ŗ»ÆµÄ·“Ó¦ČČĻąµ±Š”¶ųĒŅ×Ŗ»ÆĖŁĀŹ½ĻĀż£¬ÓŠŹ±»¹ŗܲ»ĶźČ«£¬²ā¶Ø·“Ó¦ČČŗÜĄ§ÄŃ”£ĻÖŌŚæÉøł¾ŻøĒĖ¹Ģį³öµÄ”°²»¹Ü»Æѧ¹ż³ĢŹĒŅ»²½Ķź³É»ņ·Ö¼ø²½Ķź³É£¬Õāøö×ܹż³ĢµÄČČŠ§Ó¦ŹĒĻąĶ¬µÄ”±¹ŪµćĄ“¼ĘĖć·“Ó¦ČČ”£ŅŃÖŖ£ŗ
P4(°×Į×£¬s)£«5O2(g)===P4O10(s) ¦¤H1£½£2 983.2 kJ·mol£1¢Ł
P(ŗģĮ×£¬s)£«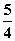 O2(g)===
O2(g)===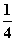 P4O10(s) ¦¤H2£½£738.5 kJ·mol£1¢Ś
P4O10(s) ¦¤H2£½£738.5 kJ·mol£1¢Ś
Ōņ°×Į××Ŗ»ÆĪŖŗģĮ×µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________”£ĻąĶ¬×“æöĻĀ£¬ÄÜĮæדĢ¬½ĻµĶµÄŹĒ________£»°×Į×µÄĪČ¶ØŠŌ±ČŗģĮ×________(Ģī”°øß”±»ņ”°µĶ”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
2H2O(l)===2H2(g)£«O2(g) ¦¤H£½571.6 kJ·mol£1
2H2(g)£«O2(g)===2H2O(g) ¦¤H£½£483.6 kJ·mol£1
µ±1 gŅŗĢ¬Ė®±äĪŖĘųĢ¬Ė®Ź±£¬¶ŌĘäČČĮæ±ä»ÆµÄĻĀĮŠĆčŹö£ŗ¢Ł·ÅČČ£»¢ŚĪüČČ£»¢Ū2.44 kJ£»
¢Ü4.88 kJ£»¢Ż88 kJ”£ĘäÖŠÕżČ·µÄŹĒ(””””)
A£®¢ŚŗĶ¢Ż B£®¢ŁŗĶ¢Ū C£®¢ŚŗĶ¢Ü D£®¢ŚŗĶ¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĆŗČ¼Éյķ“Ó¦ČČæÉĶعżŅŌĻĀĮ½øöĶ¾¾¶Ą“ĄūÓĆ£ŗ
a.ĄūÓĆĆŗŌŚ³ä×ćµÄæÕĘųÖŠÖ±½ÓČ¼ÉÕ²śÉśµÄ·“Ó¦ČČ£»
b.ĻČŹ¹ĆŗÓėĖ®ÕōĘų·“Ó¦µĆµ½ĒāĘųŗĶŅ»Ńõ»ÆĢ¼£¬Č»ŗóŹ¹µĆµ½µÄĒāĘųŗĶŅ»Ńõ»ÆĢ¼ŌŚ³ä×ćµÄæÕĘųÖŠČ¼ÉÕ”£ÕāĮ½øö¹ż³ĢµÄČČ»Æѧ·½³ĢŹ½ĪŖ
a.C(s)+O2(g)====CO2(g) ¦¤H=E1 ¢Ł
b.C(s)+H2O(g)====CO(g)+H2(g) ¦¤H=E2 ¢Ś
H2(g)+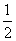 O2(g)====H2O(g) ¦¤H=E3 ¢Ū
O2(g)====H2O(g) ¦¤H=E3 ¢Ū
CO(g)+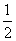 O2(g)====CO2(g) ¦¤H=E4 ¢Ü
O2(g)====CO2(g) ¦¤H=E4 ¢Ü
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÓėĶ¾¾¶aĻą±ČĶ¾¾¶bÓŠ½Ļ¶ąµÄÓÅµć£¬¼“____________________________”£
(2)ÉĻŹöĖÄøöČČ»Æѧ·½³ĢŹ½ÖŠµÄ·“Ó¦__________________ÖŠ¦¤H>0”£
(3)µČÖŹĮæµÄĆŗ·Ö±šĶعżŅŌÉĻĮ½Ģõ²»Ķ¬µÄĶ¾¾¶²śÉśµÄæÉĄūÓƵÄ×ÜÄÜĮæ¹ŲĻµÕżČ·µÄŹĒ____________”£
A.a±Čb¶ą B.a±ČbÉŁ C.aÓėbŌŚĄķĀŪÉĻĻąĶ¬
(4)øł¾ŻÄÜĮæŹŲŗć¶ØĀÉ£¬E1”¢E2”¢E3”¢E4Ö®¼äµÄ¹ŲĻµĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com