
 ��ѧ���ϵ��з���ʹ�ã�Ϊ����̫������Դ��Ѱ�÷�չ���¶����ṩ����֧�ţ����������ѧ֪ʶ�ش�
��ѧ���ϵ��з���ʹ�ã�Ϊ����̫������Դ��Ѱ�÷�չ���¶����ṩ����֧�ţ����������ѧ֪ʶ�ش����� ��1������28��Ԫ�أ�ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д��̬��ԭ�ӵ���Χ�����Ų�ʽ��
��2����̼����[Ni��CO��4]Ϊ��ɫ�ӷ���Һ�壬�۵�-25�棬�е�43�棬�۷е�ͣ�������ˮ���������л��ܼ���Ӧ���ڷ��Ӿ��壻������ΪCO��ԭ����������ͬ���۵�������Ҳ��ͬ��������Ϊ�ȵ����壻
��3��NH4F�����ӻ����笠������к��й��ۼ�����λ����N��F��H����Ԫ�صĵ縺�ԣ�F��N��H����NF3�й��õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�
��4����ͬһ����Ԫ�صĵ�һ����������ԭ�������������������VA��Ԫ�ص�һ�����ܴ��ڵ�VIAԪ�أ�
�ڼ���Seԭ�ӹµ��Ӷ������۲���Ӷ���������ȷ����ռ乹�ͣ�
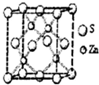
����ͼ��֪Znԭ����λ��Ϊ4�����ݾ�̯�����㾧����Zn��Sԭ����Ŀ���ð���٤��������ʾ�������������ٽ��m=��V���㣮
��� �⣺��1������28��Ԫ�أ�ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]3d84s2����Χ�����Ų�ʽΪ3d84s2���ʴ�Ϊ��3d84s2��
��2����̼�����۷е�ϵͣ��������л��ܼ������ڷ��Ӿ��壬CO��N2�����ж�����2��ԭ�ӣ����������ֱ�Ϊ14��14����Ϊ�ȵ����壬��Ϊ�����Ӿ��壻N2��
��3��NH4F�����ӻ����笠������к��й��ۼ�����λ����N��F��H����Ԫ�صĵ縺�ԣ�F��N��H����NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3������Cu2+�γ������ӣ�
�ʴ�Ϊ�����Ӽ������ۼ�����λ������F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3������Cu2+�γ������ӣ�
��4����ͬһ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�Asԭ��4p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ������As��Se���ʴ�Ϊ������
�ڶ�������������Seԭ�ӹµ��Ӷ���=$\frac{6-2��2}{2}$=1���۲���Ӷ���=2+1=3��������ռ乹����V�Σ��ʴ�Ϊ��V�Σ�
�۸���ͼƬ֪��ÿ��Zn��������4��S���ӣ���������λ����4��
������Znԭ����ĿΪ4��Sԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����������Ϊ4��$\frac{65+32}{{N}_{A}}$g���������V=��a��10-10 cm��3����4��$\frac{65+32}{{N}_{A}}$g=��a��10-10 cm��3����g/cm3�������ɵ�NA=$\frac{388��1{0}^{30}}{��{a}^{3}}$��
�ʴ�Ϊ��$\frac{388��1{0}^{30}}{��{a}^{3}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����������������ʡ��ȵ����塢��λ���������ܡ��ռ乹�͡���������ȣ�ע������ͬ����Ԫ�ص�һ�������쳣ԭ��3���и��ݵ縺��������λ���γ����ף�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ����NO2��Cl2 | B�� | һ����SO2 ��NO | ||
| C�� | ������NO2 | D�� | һ����SO2��������NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ���� | B�� | ��Na0H��Һ����� | C�� | ��Na2SO4��Һ | D�� | ֱ�ӹ۲� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
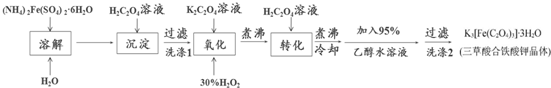
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
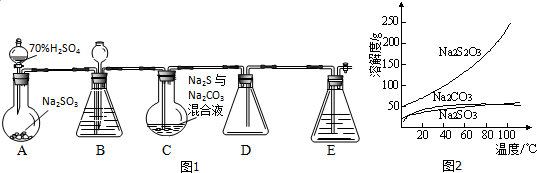
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | ���� | |||
| H-H | 436 | Cl-Cl | 243 | H-Cl | 432 |
| S=S | 255 | H-S | 339 | C-F | 427 |
| C-Cl | 330 | C-I | 218 | H-F | 565 |
| C-O | 347 | H-O | 464 |
| A�� | ����Խ�����ʵ����ȶ���Խǿ | |
| B�� | �Ȼ�ѧ����ʽH2��g��+Cl2��g���T2HCl��g������H=-QkJ•mol-1�У�Q��ֵΪ185 | |
| C�� | H-O���ļ��ܴ���H-S���ļ��ܣ�����H2O���ܷе����H2S���ܷе� | |
| D�� | C-Br���ļ���Ӧ��218kJ•mol-1-330kJ•mol-1֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �״� | B�� | ��Ȳ | C�� | �Ҵ� | D�� | ��ȩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | K��Na | B�� | ���ʯ��SiO2 | C�� | SiH4��CH4 | D�� | KCl��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ�ص���������ϼ�����ֵ��һ�����������ڵ������� | |
| B�� | ͬ��������Ԫ�ص�ԭ�Ӱ뾶ԽС��Խ�ѵõ����� | |
| C�� | �����ܴ��廯����Һ���û����嵥�� | |
| D�� | ���ԣ�HClO4��H2SO4��H3PO4��H2SiO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com