
��14�֣�ʵ���ҿ����ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ���£�
2KMnO4 +16HCl(Ũ) 2KCl + 2MnCl2 + 5Cl2�� +8H2O
��1���õ����ŷ��������ת�Ƶķ������Ŀ��
��2���÷�Ӧ�е��������뻹ԭ�����ʵ���֮���� ��
��3��KMnO4�������Ա�Cl2�������� ��ѡ�ǿ������������
��4���練Ӧ��ת����2mol���ӣ��������Cl2�ڱ�״�������Ϊ L��
��5��ijͬѧ����KMnO4��������100 mL0.5mol.L-1����Һ���ش��������⣺
������KMnO4��Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ�� �� ��
��Ӧ��������ƽ��ȡKMnO4���� g��
�۲��淶��ʵ������ᵼ��ʵ���������������в�����ʵ������Ӱ��ƫС���ǣ�������ţ� ��
| A����ˮ����ʱ���ӿ̶��� |
| B������ƿ�ڱڸ���ˮ���δ���ﴦ�� |
| C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���� |
| D�����ܽ������������Һ�彦���ձ��� |
��1��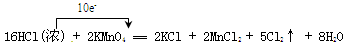 ��2��1:5 ��3��ǿ��4��22.4
��2��1:5 ��3��ǿ��4��22.4
��5���ٽ�ͷ�ι� 100mL����ƿ ��1+1�֣���7.9g ��CD ������ÿ��2�֣���14�֣�
���������������1���õ����ŷ��������ת�Ƶķ������Ŀ��Ҫע����Ӵӻ�ԭ��ָ��������������ֻ�������ӵĸ�����ע����Ӧԭ�ӵĸ�������2���÷�Ӧ��������ǻ�ԭ������������ã��ӻ��ϼ۱仯������ֻ��10����ԭ�ӵĻ��ϼ۸ı��ˣ�����10����HCl����ԭ�������������뻹ԭ�����ʵ���֮����1:5����3������������ԭ��Ӧ����������������Ҫǿ����������������ԣ�����KMnO4�������Ա�Cl2��������Ҫǿ����4�����������������ʵ���Ϊn����
2KMnO4 +16HCl(Ũ) 2KCl + 2MnCl2 + 5Cl2�� +8H2O 10e-
5 10
n 2 n=1mol V(Cl2)=1mol��22.4L/mol=22.4L��5������Һ�����ǻ�Ҫ�õ���ͷ�ιܺ�����ƿ������ƿע��Ҫ�����KMnO4����������m="n��M=c��v��M=0.5" mol.L-1��0.1 L��158g/mol=7.9g�۸��ӻᵼ����Һ�������С��Ũ�Ⱦͱ���ڶ��ݵ�ʱ��Ҫ��ˮ����������ƿ�ڱڸ���ˮ���Ũ��û��Ӱ�죻�ߵ�ҡ����Һ��������仯�����ڷ��Ӽ���������ɵģ��ټ�ˮ��ʹ����Һ�������Ũ�ȱ�С���ܽ������������Һ�彦���ձ���������������ʧ��Ũ�ȱ�С��ѡCD��
���㣺������ԭ��Ӧ�ĸ���͵��ӵ�ת�Ƽ��㡢һ�����ʵ���Ũ����Һ�����ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ϊ���ȫ����Դ�뻷�����⣬���ܼ����ѳɹ�ʶ�����д�ʩ�����ڽ��ܼ��ŵ���
| A�����С�����һСʱ��Ϩ�ƻ |
| B��¶����յ��ݺͽո� |
| C�����콫�յ����¶�������26������ |
| D����������������մ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
14��)
��һ�������й�ʵ��������жϲ���ȷ���� ������ţ���
A������һ�����ʵ���Ũ����Һ������ʱƽ�ӿ̶��ߡ�
B���������Ȼ�̼���Ҵ������л��ܼ�����������ȡ��ˮ�е��塣
C����һ����ͨ�����壬�Ӵ�ֱ�ڹ��ߵķ�����Կ���һ�������ġ�ͨ·����
D������100mL1mol/L��NaOH��Һ�������4g���������ƹ��塣
E���ò�˿պȡ������Һ����ɫ��Ӧ,û�й۲쵽��ɫ�����Ը���Һ�в�����Ԫ�ء�
������ʵ������Ҫ480mL0.1mol/LNa2CO3��Һ������̼���ƾ������ƣ��ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ��̼���ƣ�Na2CO3��10H2O������ g��
��2����ʵ�黹��Ҫ�������� ��
��3����ʵ�����������,��Һ��Ũ�Ƚ��ᣨ��ƫ�ߡ�ƫ�͡�����)
A����ˮ����ʱ���ӿ̶��� ��
B������ƿ�ڱڸ���ˮ���δ���ﴦ�� ��
C��������̼���ƾ��岿��ʧ�ᾧˮ ��
��4����������ѱ�ǩ�ϵ�����дһ��(��ͼ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������NaOH��������250mL 1��25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������ƿ����������������е� ����3�֣�
���¶� ��Ũ�� ������ ��ѹǿ �ݿ̶���
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ�� ����3�֣�
����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
�۽�����ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
�ܽ�����ƿ�ǽ����ߵ�ҡ��
�ݸ��ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
����ʱ�������Ҫʵ�������� ��5�֣�
��4����6�֣����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
����ֽ����NaOH���塣 ____________;
��������ƿ��ˮ���ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ�����ȡ�κδ�ʩ��___;
��������NaOH��Һ�������ձ��ڡ�____________;
��������ƿ��ˮʱ�۾�һֱ����Һ�档____________;
������ǰ������ƿ������������ˮ��____________;
����NaOHʱ��������������̡�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�(ʵ����)��ͼΪ����250 mL 0��2 mol/L Na2CO3��Һ��ʾ��ͼ��
�ش��������⣺
��1�����гƵ�Na2CO3________g��ѡȡ����ƿ���______________
��2������ƿʹ��ǰ����©ˮ�ķ����� ____________________��
��3�������������������������ҺŨ���к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
| A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ����________�� |
| B��û�н��в�������ܺ͢�________�� |
| C���ڵڢݲ�����������Һ����������ƿ�� ________�� |
| D��δ����ȴ���Ƚ���Һע������ƿ�ж��� ________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣�ʵ����Ҫ����480 mL 0.2 mol/L NaOH��Һ����ش��������⣺
��1�����ƹ���������Ҫʹ�õĻ�ѧ������__________________����ѡ�����ĸ����
A���ձ� B��500 mL����ƿ C��©�� D����ͷ�ι� E��������
��2����������ƽ��ȡ�������ƣ�������Ϊ_______________g��
��3��ȡ����������ĸ�NaOH��Һʱ�������������в�����ȡ����Ķ��ٶ��仯����_______��
A����Һ��NaOH�����ʵ��� B����Һ��Ũ��
C����Һ��OH������Ŀ D����Һ���ܶ�
��4��������������Һ�Ĺ����У���������� NaOH��Һ���ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�������Ӱ�족��
��δ����ȴ���Ƚ���Һע������ƿ�У�______________
������ƿ������ϴ�Ӻ������������ˮ��_____________
�۶���ʱijͬѧ�۲�Һ��������ͼ��ʾ���������Ƶ���Һ��Ũ��____________��
��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���___________������ƫ�͡�������Ӱ�족��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣�ʵ����Ϊ���ijһʵ�飬����240mL1.0mol/L��ϡ���ᣬ����11.8mol/L��Ũ��������,��ղ���ش��������⣺
��1���������ijһʵ������1.0mol/L��������Һ��Ӧ��ȡŨ�������Ϊ mL�����õ��IJ�����������Ͳ���ձ����������Ҫ�õ��� �� �ȡ�
��2������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ���ġ�
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
F������ij������ˮ��ʹ��Һ��Һ��ǡ����̶�����
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨ƫ�ߡ�ƫ�ͻ���Ӱ�죩��û�н���A���� ���ò���ʱ����������Һ�彦������ƿ�� ������ʱ����_____________��
��4��10.6gNa2CO3�����������Ƶ����ᷴӦ������CO2�����ڱ�״���µ������__________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
19��)ʵ�������õ�������250 mL0.2 mol��L-1��CuSO4��Һ��
��1��������Һʱ��һ����Է�Ϊ���¼������裺(������пո�)
A ����B ���� C���ܽ�D��______E. ______F. ���� G. ҡ�ȡ�װƿ
��2����ʵ������õ�����������ƽ��ҩ�ס����������ձ�����Ͳ����______
��______ �����������ƣ�
��3����������Ҫ��ȡCuSO4��5H2O������Ϊ______ ��
��4������ȡ����ʱ���뱻��������(10g����������)�������Ƶ�CuSO4��Һ��Ũ��______ (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��������ʱ���ӿ̶��ߣ������Ƶ�CuSO4��Һ��Ũ��______ (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��
��5����ȷ���ƺõ�CuSO4��Һ��ȡ��50mL ������50mL CuSO4��Һ�����ʵ���Ũ��Ϊ______________������Cu2+������Ϊ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com