
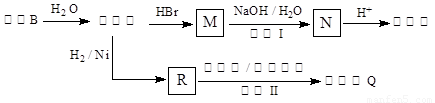
����������ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������������H�ĺϳ�·�ߣ�
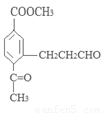
��1��ԭ��A��ͬ���칹���У����б����Һ˴Ź�����������5����Ľṹ��ʽΪ ��
д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ��
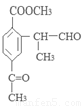
��2���ڵķ�Ӧ������ ��ԭ��D�к��еĹ����������� �� ��
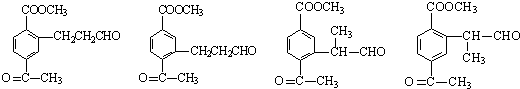
��3��д�����������������м����F��ͬ���칹��Ľṹ��ʽ ��
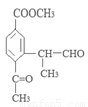
(i) �ܷ���������Ӧ��
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3��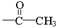 ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ��
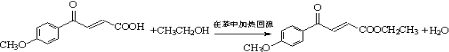
��4��ԭ��B�������������������������ᣨHOOC-CH=CH-COOH�����������������ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·�ߡ��ϳ�·������ͼʾ�����£�
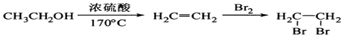

��������
�����������1��ԭ��A�ķ���ʽΪC7H8O�����ĺ��б����Һ˴Ź�����������5�����ͬ���칹��ṹ��ʽΪ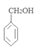 ��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ
��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹��
����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹��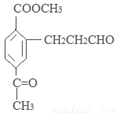 ��
�� ��
�� ��
��
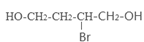
��4����ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·��Ϊ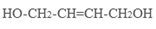 +HBr
+HBr
 ��
��
 +O2
+O2
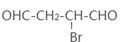 ��
��
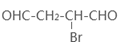 +O2
+O2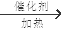
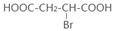 ��
��
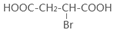 +NaOH
+NaOH
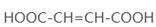 +NaBr+H2O��
+NaBr+H2O��
���㣺�����л�������š��ṹ��ʽ��ͬ���칹�����д���ϳɵ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʣ�������ij�о�С�������һ��������
����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʣ�������ij�о�С�������һ�������� �����H�ĺϳ�·�ߣ�
�����H�ĺϳ�·�ߣ�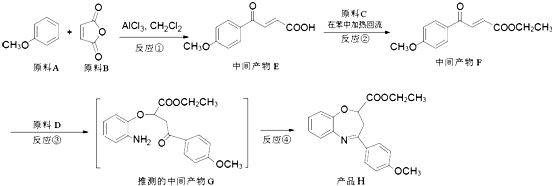



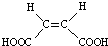 ���������������Ծ����б仯�ֱ�õ�ƻ���ᣨ
���������������Ծ����б仯�ֱ�õ�ƻ���ᣨ ���;ۺ���Q��
���;ۺ���Q��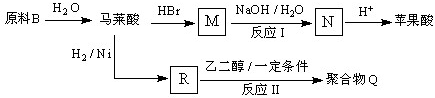
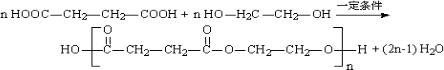
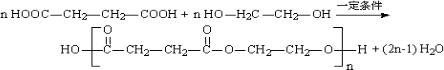
 ������дһ�֣�
������дһ�֣� ������дһ�֣�
������дһ�֣� ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004ȫ����ʡ�и߿�ģ�������ࡤ��ѧ ���ͣ�038
��ը�����������˲�䷢���������͵Ĺ��̣�
(1)��ƽ�ڻ�ҩ������ըʱ�Ļ�ѧ����ʽ��
____KNO3(s)��____C(s)��____S(s)��____K2S(s)��____CO2(g)��____N2(g)
(2)������������(��ѧʽ��C8N8O16����Է���������464)���������ƹ����е�һ��������ըҩ�������ܶ�ԼΪ2g��cm��3��������ըʱ��Ӧ�Ļ�ѧ����ʽΪ��
C8N8O16(s)��8CO2(g)��4N2(g)
�Թ�������������鱬ըʱ������Ϊ��״��ʱ���ʵ������ԭ���ʵĶ��ٱ���(ȡ����ֵ)
(3)��ѹ�����£�һ����������������¶ȱ仯��ϵΪ��
 ��
�� [ʽ��Tָ�����±꣬T(K)��273��t C]
[ʽ��Tָ�����±꣬T(K)��273��t C]
��ըҩ��ը��������¶ȴﵽ2730Kʱ�����������DZ�״���µĶ��ٱ���
(4)���������������������ΪӰ��ըҩ�ı�ը���ܵ������ǣ�
________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���к�����������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
������ ����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ��������
����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������� �����H�ĺϳ�·�ߣ�
�����H�ĺϳ�·�ߣ�
��1��ԭ��A��ͬ���칹���У����б������Һ˴Ź�����������4������� ��д����ṹ��ʽ����
��2����Ӧ�ڵĻ�ѧ����ʽ��_________________��
��3���۵ķ�Ӧ������___________��ԭ��D�к��еĹ�����������_________��_________��
��4��ԭ��B�������������������������ᣨ˳��ϩ���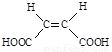 ����������
����������
�����Ծ����б仯�ֱ�õ�ƻ���ᣨ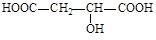 ���;ۺ���Q��
���;ۺ���Q��
д����ӦI�ͷ�ӦII�Ļ�ѧ����ʽ��___________________��_____________________��
��5�����������������м����F��ͬ���칹����Ŀ��________�������������칹����д����������һ�ֵĽṹ��ʽ___________��
(i) �ܷ���������Ӧ��
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3��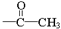 ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ڽ��и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
�����ӻ�����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ����������� ����ȱѪ�����ʡ�������ij�о�С�������һ�������ӻ������H�ĺϳ�·�ߣ�
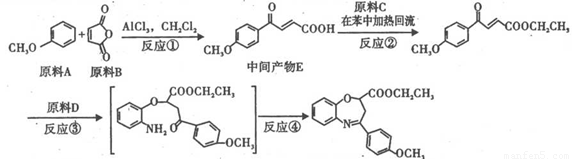
(1)ԭ��A��ͬ���칹���У����б������Һ˴Ź�����������4�������______(д����ṹ��ʽ����
(2)�۵ķ�Ӧ������______��ԭ��D�к��еĹ�����������______��______��
(3)��Ӧ�ڵĻ�ѧ����ʽ��______________________________��
(4)ԭ��B��������������,����ÿ����(˳��ϩ���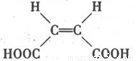 ��������,�����Ծ����б仯�ֱ�õ�ƻ���ᣨ
��������,�����Ծ����б仯�ֱ�õ�ƻ���ᣨ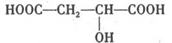 ���;ۺ���Q:
���;ۺ���Q:
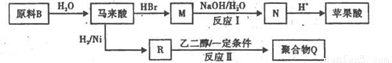
�ٰ뷽����ԭ��B��ͬ���칹�壬�����к�1����(��Ԫ̼������1���ǻ��������� ��O��O�������뷽��Ľṹ��ʽ��____________��
��д����ӦI�ͷ�ӦII�Ļ�ѧ����ʽ_____________��____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com