
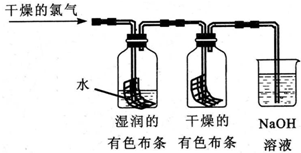
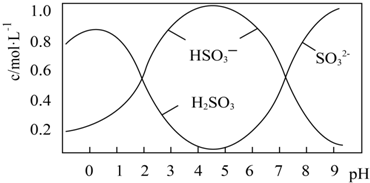
·ÖĪö ŅŃÖŖCl2+H2O?HCl+HClO£¬ĀČĘų¾ßÓŠĘư׊ŌŹĒŅņĪŖĘäÓėĖ®·“Ӧɜ³ÉµÄHClO¾ßÓŠĘư׊Ō£¬ĖłŅŌøÉŌļµÄCl2²»ÄÜŹ¹ÓŠÉ«²¼ĢõĶŹÉ«£¬
£Ø1£©ĶØČėøÉŌļµÄĀČĘųŗ󣬼ÆĘųĘæÖŠŹŖČóµÄÓŠÉ«²¼ĢõŗÜæģĶŹÉ«£¬¶ųøÉŌļµÄÓŠÉ«²¼ĢõƻӊĶŹÉ«£¬ĖµĆ÷ĀČĘųĪŽĘư׊Ō£»
£Ø2£©ĻøŠÄµÄŅŅĶ¬Ń§·¢ĻÖ£¬Ņ»¶ĪŹ±¼äŗóøÉŌļµÄÓŠÉ«²¼ĢõŅ²ĶŹÉ«ĮĖ£¬ĖµĆ÷Ė®ÕōĘų½ųČė·¢Éś·“Ӧɜ³ÉĮĖ“ĪĀČĖį£¬ŠčŅŖŌŚøÉŌļµÄÓŠÉ«²¼ĢõĒ°Ģķ¼ÓøÉŌļ×°ÖĆ£»
£Ø3£©ŅŅĶ¬Ń§Ö¤Ć÷øÉŌļĀČĘųĪŽĘư׊Ō£¬ĀČĘųŗĶĖ®·“Ӧɜ³É“ĪĀČĖįŗĶŃĪĖį£¬ČÜŅŗÖŠ“ęŌŚ“ĪĀČĖįøłĄė×Ó¶¼æÉÄܶ¼¾ßÓŠĘư׊Ō£¬½«ŃĪĖįµĪµ½ÓŠÉ«²¼ĢõÉĻ£¬·¢ĻÖÓŠÉ«²¼Ģõ²»ĶŹÉ«ĖµĆ÷²»ŹĒHClµÄĘư׊Ō£¬½«“ĪĀČĖįÄĘČÜŅŗµĪČėµ½ÓŠÉ«²¼Ģõ²»ĶŹÉ«ĖµĆ÷²»ŹĒ“ĪĀČĖįøłĄė×ÓĘšµÄ×÷ÓĆ£¬“Ó¶ųÖ¤Ć÷Ęư׊ŌŹĒ“ĪĀČĖįµÄ×÷ÓĆ£®
½ā“š ½ā£ŗ£Ø1£©ĶØČėøÉŌļµÄĀČĘųŗ󣬼ÆĘųĘæÖŠŹŖČóµÄÓŠÉ«²¼ĢõŗÜæģĶŹÉ«£¬¶ųøÉŌļµÄÓŠÉ«²¼ĢõƻӊĶŹÉ«£¬Ņņ“Ė¼×Ķ¬Ń§µĆ³ö½įĀŪ£ŗøÉŌļµÄĀČĘųƻӊĘư׊Ō£¬³±ŹŖµÄĀČĘųÓŠĘư׊Ō£¬
¹Ź“š°øĪŖ£ŗøÉŌļµÄĀČĘųƻӊĘư׊Ō£¬³±ŹŖµÄĀČĘųÓŠĘư׊Ō£»
£Ø2£©ĻøŠÄµÄŅŅĶ¬Ń§·¢ĻÖ£¬Ņ»¶ĪŹ±¼äŗóøÉŌļµÄÓŠÉ«²¼ĢõŅ²ĶŹÉ«ĮĖ£¬Ėū¾¹ż×ŠĻøĖ¼æ¼£¬ČĻĪŖ¼×µÄÉč¼ĘӊȱĻŻ£¬Ė®ÕōĘų»įĖęĀČĘų½ųČėøÉŌļµÄÓŠÉ«²¼ĢõµÄ¼ÆĘųĘ棬ĀČĘųŗĶĖ®·“Ӧɜ³ÉŃĪĖįŗĶ“ĪĀČĖį¾ßÓŠĘÆ°××÷ÓĆ£¬²¼ĢõĶŹÉ«£¬×°ÖĆÓ¦Ó¦øĆ×öČēĻĀŠŽøÄ£ŗŌŚŹŖČóµÄ²¼ĢõŗĶøÉŌļµÄ²¼ĢõÖ®¼äĮ¬½ÓŅ»øöøÉŌļ×°ÖĆ£¬
¹Ź“š°øĪŖ£ŗŌŚŹŖČóµÄ²¼ĢõŗĶøÉŌļµÄ²¼ĢõÖ®¼äĮ¬½ÓŅ»øöøÉŌļ×°ÖĆ£»
£Ø3£©ŅŅĶ¬Ń§ŹµŃéÉč¼ĘŹĒÖ¤Ć÷øÉŌļĀČĘųĪŽĘư׊Ō£¬ŹŖČóµÄĀČĘų¾ßÓŠĘư׊Ō£¬ĀČĘųŗĶĖ®·“Ӧɜ³É“ĪĀČĖįŗĶŃĪĖį£¬ČÜŅŗÖŠ“ęŌŚ“ĪĀČĖįøłĄė×Ó£¬ĖłŅŌÉś³ÉµÄHCl”¢HClO”¢ClO-¶¼æÉÄܶ¼¾ßÓŠĘư׊Ō£¬½«ŃĪĖįµĪµ½ÓŠÉ«²¼ĢõÉĻ£¬·¢ĻÖÓŠÉ«²¼Ģõ²»ĶŹÉ«ĖµĆ÷²»ŹĒHClµÄĘư׊Ō£¬½«“ĪĀČĖįÄĘČÜŅŗµĪČėµ½ÓŠÉ«²¼Ģõ²»ĶŹÉ«ĖµĆ÷²»ŹĒ“ĪĀČĖįøłĄė×ÓĘšµÄ×÷ÓĆ£¬“Ó¶ųÖ¤Ć÷Ęư׊ŌŹĒ“ĪĀČĖįµÄ×÷ÓĆ£¬Ņņ“ĖŌö¼ÓµÄŹµŃéŹĒ£ŗ“ĪĀČĖįÄĘČÜŅŗ»ņ“ĪĀČĖįøĘČÜŅŗµĪ¼Óµ½ÓŠÉ«²¼ĢõÉĻ£¬ČōÓŠÉ«²¼Ģõ²»ĶŹÉ«£¬ŌņÖ¤Ć÷HClOÓŠĘư׊Ō£¬
¹Ź“š°øĪŖ£ŗ“ĪĀČĖįÄĘČÜŅŗ»ņ“ĪĀČĖįøĘČÜŅŗµĪ¼Óµ½ÓŠÉ«²¼ĢõÉĻ£¬ČōÓŠÉ«²¼Ģõ²»ĶŹÉ«£¬ŌņÖ¤Ć÷HClOÓŠĘư׊Ō£»
µćĘĄ ±¾Ģāæ¼²éĮĖĀČĘų¼°Ęä»ÆŗĻĪļŠŌÖŹ·ÖĪö”¢·“Ó¦ĻÖĻóµÄĄķ½āÓ¦ÓĆ£¬Ö÷ŅŖŹĒŃéÖ¤“ĪĀČĖįĘư׊ŌµÄŹµŃéÉč¼Ę£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe”¢Al”¢CuæÉŅŌ·Ö±šÓĆÖĆ»»·Ø”¢Ö±½Ó¼ÓČČ·ØŗĶµē½ā·ØŅ±Į¶µĆµ½ | |
| B£® | ŗ¬øĘ”¢±µ”¢²¬µČ½šŹōŌŖĖŲµÄĪļ֏ӊѤĄöµÄŃÕÉ«£¬æÉÓĆÓŚÖĘŌģŃ껚 | |
| C£® | ŹÆÓĶĮŃ½ā”¢ĆŗµÄĘų»Æ”¢ŗ£Ė®ÖĘĆ¾¶¼°üŗ¬»Æѧ±ä»Æ | |
| D£® | ¹¤ŅµÉĻĪŖĮĖ¼Óæģ·ÖĄė½ŗĢåÖŠµÄµē½āÖŹŌÓÖŹ£¬³£ŌŚÉųĪö“üĶāŹ©¼Óµē³”£¬Ź¹µē½āÖŹĄė×ÓĶø¹ż°ėĶøĤĻņĮ½¼«ŅĘ¶Æ£¬øĆ²Ł×÷ŹĒÓ¦ÓĆ½ŗĢåµÄµēÓ¾ŌĄķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
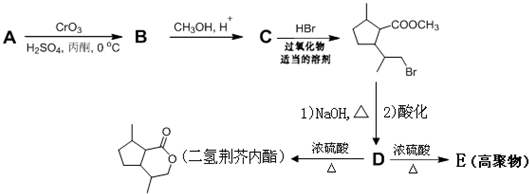
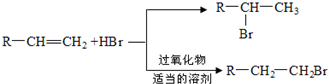
 £¬DÖŠŗ¬ÓŠµÄ¹ŁÄÜĶŵÄĆū³ĘōČ»ł”¢ōĒ»ł£®
£¬DÖŠŗ¬ÓŠµÄ¹ŁÄÜĶŵÄĆū³ĘōČ»ł”¢ōĒ»ł£®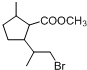 +2NaOH$\stackrel{”÷}{”ś}$
+2NaOH$\stackrel{”÷}{”ś}$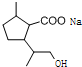 +CH3OH+NaBr£®
+CH3OH+NaBr£®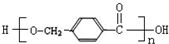 £©µÄĀ·Ļß²¹Č«ĶźÕū
£©µÄĀ·Ļß²¹Č«ĶźÕū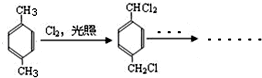
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
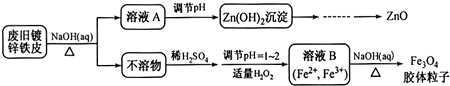
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
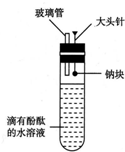 ÓŠČĖÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬ÄæµÄŹĒ×öÄĘÓėĖ®·“Ó¦µÄŹµŃé²¢ŃéÖ¤£ŗ
ÓŠČĖÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬ÄæµÄŹĒ×öÄĘÓėĖ®·“Ó¦µÄŹµŃé²¢ŃéÖ¤£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | KClO3£ØMnO2£¬¼ÓČČ£© | B£® | KMnO4£Ø¼ÓČČ£© | C£® | H2O2£ØMnO2£© | D£® | HgO£Ø¼ÓČČ£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
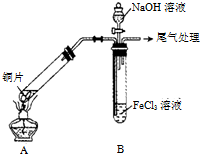 ijŠ£»ÆѧŠĖȤŠ”×éĢ½¾æSO2ÓėFeCl3ČÜŅŗµÄ·“Ó¦£¬ĖłÓĆ×°ÖĆČēĶ¼ĖłŹ¾£Ø¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©£®
ijŠ£»ÆѧŠĖȤŠ”×éĢ½¾æSO2ÓėFeCl3ČÜŅŗµÄ·“Ó¦£¬ĖłÓĆ×°ÖĆČēĶ¼ĖłŹ¾£Ø¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æŖŹ¼Īö³ö¾§ĢåŗóÓĆ²£Į§°ō½Į°č | |
| B£® | ĀĖČ„²»ČÜŠŌŌÓÖŹŗ󣬽«ĀĖŅŗŅĘÖĮŪįŪöÄŚ¼ÓČČÅØĖõ | |
| C£® | µ±Õō·¢µ½Ź£ÓąÉŁĮæŅŗĢåŹ±£¬Ķ£Ö¹¼ÓČČ£¬ĄūÓĆÓąČČ½«ŅŗĢåÕōøÉ | |
| D£® | ½«ÖĘµĆ¾§Ģå×ŖŅʵ½ŠĀÖĘ¹żĀĖĘ÷ÖŠÓĆ“óĮæĖ®½ųŠŠĻ“µÓ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com