�̷����壨FeSO
4?7H
2O����ҽҩ������Ѫ����ijͬѧ��KMnO
4��Һ�ζ��̷����壨FeSO
4?7H
2O����Ʒ�����ʲ��� KMnO
4��Ӧ��������Ԫ�غ������вⶨ��
��1���۲���Ʒʱ�����ֱ�������һ���ɫ���ʣ�������ʲô����
Fe2��SO4��3
Fe2��SO4��3
���ѧʽ��
��2��ʵ��ʱ�õ�����Ҫ�������ζ��ܡ��ζ��ܼС�����̨���ձ��⣬����
��ƿ
��ƿ
��
��3���ζ�ʱ����Ӧ�����ӷ���ʽ
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
��
��4������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������
b
b
��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
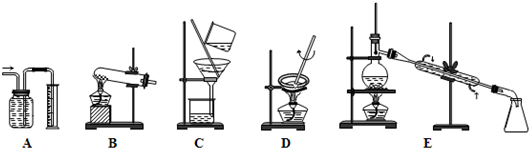
��5��KMnO4��Һ Ӧװ��
��
��
ʽ�ζ����У�����ζ����յ�ʱ��־��
��������һ��KMnO4��Һ����Ӧ����Һ����ɫΪ��ɫ���Ұ���Ӳ���ɫ��
��������һ��KMnO4��Һ����Ӧ����Һ����ɫΪ��ɫ���Ұ���Ӳ���ɫ��
��
��6������˵����ȷ����
D
D
��
A��ѡ�ù��Ϊ25.00ml�ĵζ���װ KMnO
4��Һ���ζ� ʱ��ȥ20.00ml��Һ��ʣ����ҺΪ5.00ml��
B���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�ζ�����Һ��ı仯��
C�����Ʊ�ҺKMnO
4ʱ�����ݸ��ӣ���ʹ�ⶨ���ƫ�ߣ�
D����¼�ζ����ʱ���ζ�ǰ�������ӣ��ζ���������ӻ�ʹ�ⶨ���ƫ�ͣ�
��������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
��֪��CH
4��g��+H
2O��g��=CO��g��+3H
2��g����H=+206.2kJ?mol
-1CH
4��g��+CO
2��g��=2CO��g��+2H
2��g����H=+247.4kJ?mol
-1��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH
4��g����H
2O��g����Ӧ����CO
2��g����H
2��g�����Ȼ�ѧ����ʽΪ
CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+165.0kJ?mol-1
CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+165.0kJ?mol-1
��
��2���������[CO��NH
2��
2]�ļ�����Һ�����װ��ʾ��ͼ��ͼ�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ
CO��NH2��2+8OH-+6e-�TCO32-+N2��+6H2O
CO��NH2��2+8OH-+6e-�TCO32-+N2��+6H2O
�������ĵ缫��Ӧʽ
6H2O-6e-�T3H2��+6OH-
6H2O-6e-�T3H2��+6OH-
��

 �̷����壨FeSO4?7H2O����ҽҩ������Ѫ����ijͬѧ��KMnO4��Һ�ζ��̷����壨FeSO4?7H2O����Ʒ�����ʲ��� KMnO4��Ӧ��������Ԫ�غ������вⶨ��
�̷����壨FeSO4?7H2O����ҽҩ������Ѫ����ijͬѧ��KMnO4��Һ�ζ��̷����壨FeSO4?7H2O����Ʒ�����ʲ��� KMnO4��Ӧ��������Ԫ�غ������вⶨ��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�