
 2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��
2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��A��Q2=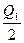 | B��Q2��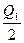 ="197" kJ ="197" kJ | C��Q2��Q1��197 kJ | D��Q1=Q2="197" kJ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH(aq)+HCl(aq)==NaCl(aq)+H2O(l)����H=" +28.7" kJ��mol-1 |
| B��NaOH(aq)+HCl(aq)==NaCl(aq)+H2O(l)����H= _28.7 kJ��mol-1 |
| C��NaOH(aq)+HCl(aq)==NaCl(aq)+H2O(l)����H="+57.4" kJ��mol-1 |
| D��NaOH(aq)+HCl(aq)==NaCl(aq)+H2O(l)����H= _57.4 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
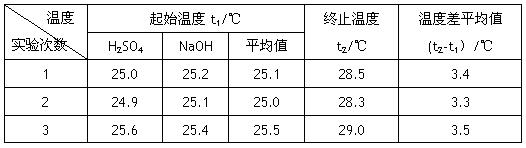
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/(KJ/mol) | -283.0 | -285.8 | -726.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ZnO(s);��H="-351.5" kJ��mol-1
ZnO(s);��H="-351.5" kJ��mol-1 HgO(s);��H="-90.84" kJ��mol-1
HgO(s);��H="-90.84" kJ��mol-1 ZnO(s)+Hg(l)�Ħ�HΪ
ZnO(s)+Hg(l)�Ħ�HΪ| A����H="+260.7" kJ��mol-1 | B����H="-260.7" kJ��mol-1 |
| C����H="-444.2" kJ��mol-1 | D����H="+444.2" kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڻ�ѧ��Ӧ���еĹ����зų������յ�������Ϊ��Ӧ�ȡ� |
| B����ϡ��Һ�У�1mol���1mol����кͷ�Ӧ���ų������������к��ȡ� |
| C���������ȷ�Ӧ����Ӧ�������е����������ǵ��������������е��������� |
| D����101Kpaʱ��1mol������ȫȼ�������ȶ�������ʱ�����ų����������������ʵ�ȼ���ȡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʹ�û��β�������Ϊ�˼ӿ췴Ӧ���ʣ���Сʵ����� |
| B��Ϊ��ȷ�ⶨ��Ӧ�����Һ���¶ȣ�ʵ�����¶ȼ�ˮ����Ӧ��С�ձ��ײ��Ӵ� |
| C����0.5mol��L��1NaOH��Һ�ֱ���0.5 mol��L��1�����ᡢ������Һ��Ӧ������ȡ����Һ�����ȣ����õ��к�����ֵ��ͬ |
| D���ڲⶨ�к���ʵ������Ҫʹ�õ������У���ƽ����Ͳ���ձ����ζ��ܡ��¶ȼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȷ�Ӧ��������ӳɼ�ʱ�ͷŵ����������ڷ�Ӧ����Ӷϼ�ʱ���յ������� |
| B�����ȷ�Ӧ��Ӧ�����������ڲ�������������H��0 |
| C�����ȷ�Ӧ���ü��ȾͿ��Խ��� |
| D���������Ϸ�Ӧ�����е�ȼ�ա�����кͷ�Ӧ���������ᷴӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ��������ˮ����̬��ΪҺ̬ | B��ˮ�����ɸߴ�����ʹ� |
| C��̼������ڳ�������·ֽ� | D��CO��ȼʱ����CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com