
˫��ˮ(H2O2)��һ��ҽ������ɱ��������֪H2O2���ӵĽṹ��ͼ��ʾ��H2O2���Ӳ���ֱ���εģ�����Hԭ�������ڰ�չ�����������ֽ�ϣ������Ϊ93��52�䣬������O��H ����O��O���ļнǾ�Ϊ96��52�䡣�Իش�
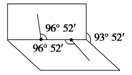
(1)H2O2���ӵĵ���ʽΪ________________���ṹʽΪ______________________��
(2)H2O2�����д���________����________����Ϊ______(����ԡ��Ǽ��ԡ�)���ӡ�
(3)H2O2������CS2����ԭ����____________________________________________
________________________________________________________________________
________________________________________________________________________��
(4)H2O2��������Ԫ�صĻ��ϼ�Ϊ__________�ۣ�ԭ����_____________________
________________________________________________________________________
________________________________________________________________________��
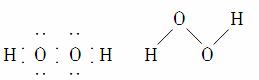
(2)���ԡ��Ǽ��ԡ����ԡ�(3)��ΪH2O2Ϊ���Է��ӣ�CS2Ϊ�Ǽ��Է��ӣ����ݡ��������ܡ�ԭ����֪H2O2������CS2��(4)��1����ΪO��O��Ϊ�Ǽ��Լ���O��H��Ϊ���Լ������õ��Ӷ�ƫ������������Ԫ���ԣ�1��
������(1)H2O2���ӵĿռ乹��Ϊ���Գƽṹ��ӦΪ���Է��ӣ�����O��O�Ǽ��Լ���O��H���Լ���(2)���ݡ��������ܡ�ԭ����֪��H2O2�����ڷǼ����ܼ�CS2��(3)���õ��ӶԵ�ƫ�ƾ�����Ԫ���ڻ������еĻ��ϼۡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȫ�ۻ�������������������Һ�У����ᷢ����Ӧ����(����)
A��ϡ���� B��ϡ���� C������ͭ D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A�����෴��ɵ�����֮����������Ϊ���Ӽ�
B������Ԫ����ǽ���Ԫ�ػ���ʱ����һ���γ����Ӽ�
C��ijԪ�ص�ԭ�������ֻ��һ�����ӣ�����±�ؽ��ʱ���γɵĻ�ѧ����һ�������Ӽ�
D���ǽ���ԭ�Ӽ�����γ����Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݼ۵��ӶԻ������ۣ��ж����з��ӻ������ӵĿռ乹�Ͳ��������ε���(����)
A��PCl3 B��H3O��
C��HCHO D��PH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������(��������ԭ��)��һ����˵���ɼ��Է�����ɵ����������ڼ��Է�����ɵ��ܼ����Ǽ��Է�����ɵ����������ڷǼ��Է�����ɵ��ܼ���������ʵ�п����á��������ܡ�ԭ��˵������(����)
��HCl������ˮ����I2������ˮ����Cl2������ˮ
��NH3������ˮ
A���٢� B���ڢ�
C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���в���ȷ����(����)
A���Ҽ��Ȧм��ص��̶ȴ��γɵĹ��ۼ�ǿ
B������ԭ��֮���γɹ��ۼ�ʱ�������һ���Ҽ�
C�����嵥���У�һ���ЦҼ��������Цм�
D��N2��������һ���Ҽ��������м�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������ж����ں����Լ��ķǼ��Է��ӵ���(����)
A��CO2��H2S B��C2H4��CH4
C��Cl2��C2H2 D��NH3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol·L��1�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ________��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol·L��1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________________��
(3)�����ͬ��pH������1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________________��
(4)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ���У�������Ϊ��������������Ϊij��Һ�м���ķ�Ӧ������ʵ�����ѡ�����ͼ���Ӧ����ĸ��ա�

(1)AlCl3��Һ�м��백ˮ������________��
(2)AlCl3��Һ�м���NaOH��Һ������________��
(3)AlCl3��MgCl2�����Һ�м���NaOH��Һ������________��
(4)����ʯ��ˮ��ͨ�����CO2________��
(5)�������������AlCl3��Һ�м������NaOH��Һ________��
(6)��������HCl��AlCl3��Һ�м��������ˮ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com