
��1��ij�¶��£�2L�����ܱ������У�
X��Y��Z�������巢����ѧ��Ӧʱ�����ʵ���
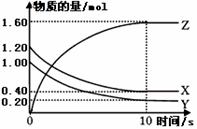 ��ʱ��仯�Ĺ�ϵ��������ͼ��ʾ����
��ʱ��仯�Ĺ�ϵ��������ͼ��ʾ����
�ٷ�Ӧ�Ļ�ѧ����ʽΪ___ _________ ____��
��0~10s�ڣ���Z��ʾ�Ļ�ѧ��Ӧ����________ ____��
��X��ת����Ϊ________ ________��
��2��֪I2��g��Ϊ�Ϻ�ɫ���ں��������У����淴ӦH2��g��+I2��g�� 2HI��g���ﵽƽ��ı�־��
2HI��g���ﵽƽ��ı�־��
A����������ܶȺ㶨���� B������������ɫ���ٸı�
C��H2��I2��HI��Ũ����� D����������ѹǿ���ٸı�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
��1���������ڵ�ij����Ԫ�أ����һ�����������������ͼ1��ʾ�����Ԫ�ض�Ӧԭ�ӵ�M������Ų�ʽΪ ��
��2������ͼ2��ʾ��ÿ�����߱�ʾ���ڱ���A-��A�е�ijһ��Ԫ���⻯��ķе�仯��ÿ��С�ڵ����һ���⻯�����a��������� ����������ж�����
��
��3��CO2�ڸ��¸�ѹ�����γɵľ����侧������ͼ3��ʾ���þ������������ ��ѡ����ӡ���ԭ�ӡ������ӡ������������塣
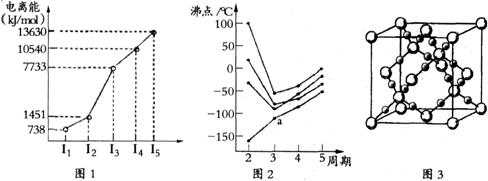
��4��BCl3ԭ�ӵ��ӻ���ʽΪ ����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����
�֡�д����BCl3�ṹ��ͬ��һ�ֵȵ����壨д���ӣ� ��
|
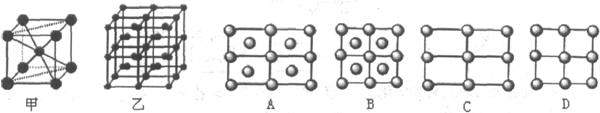
D�Ĵ����ξ���ֲ��ṹ��ͼ���þ����к��еĻ�ѧ��
��________ (��ѡ�����)��
�ټ��Լ������ڷǼ��Լ���������λ�������ܽ�����
��6��Fe��һ�־�����ס�����ʾ�����������߷������ҵõ���A-Dͼ����ȷ���� ����ԭ�ӵ���λ���� ��������ԭ�ӵİ뾶��r cm���þ�����ܶ���p g��cm3�����������ԭ������Ϊ ���谢���ӵ�������ֵΪNA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��A��B��C��D��ԭ������������������CΪ����Ԫ�أ�C��������������A��ȣ�C��D��Ԫ��ԭ�ӵ�������֮��ΪA��B��Ԫ��������֮�͵�3������ش�
(1)AΪ__________��BΪ__________��CΪ__________��DΪ__________��
(2)A��B��C��D��ԭ�Ӱ뾶��С˳��Ϊ_________________________��
(3)A��C�γɻ�����CA�ĵ���ʽ_________________________��
(4)�õ���ʽ��ʾC��D�γɻ�����C2D�Ĺ���________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ�����(S2Cl2)�ǹ㷺������ҵ����������ӽṹ��ͼ��ʾ�������£�S2Cl2��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵���������
A. S2Cl2Ϊ���ۻ�����
B. S2Cl2ֻ���м��Թ��ۼ�
C. S2Cl2��ˮ��ӦʱS��S����S��Cl��������
D. S2Cl2������S��S����S��Cl����ͨ�����õ��Ӷ��γɵ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���д�ʩ������Ӧ����������Ч����
A��Al��������ȼ������A1203����AlƬ�ij�Al�� B��Fe��ϡ���ᷴӦ��ȡH2ʱ������Ũ����
C����H2S04��BaCl2����Һ��Ӧʱ������ѹǿ D��Na��ˮ��Ӧʱ����ˮ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȼ�շ��Dzⶨ�л������ﻯѧʽ��һ����Ҫ������ȡ0.1molij����ȫȼ�գ�ȼ�ղ�������ͨ������ͼ��ʾ��װ�ã�ʵ������Ƶü�װ������5.4g����װ������26.4g��
��1����5�֣�������ķ���ʽ
��2����3�֣�����������ѧ��Ӧʹ��ˮ������KMnO4��Һ��ɫ��
д������ȴ�����ܵĽṹ��ʽ
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)������Ϊ��������H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ________________________________________________________________________��
(2)��Al������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д����������R�ĵ缫��Ӧʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼװ�ý���ʵ�飬��ʼʱ��a��b����Һ����ƽ���ܷ�ã�����һ��ʱ�䡣����˵������ȷ���� (����)
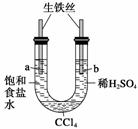
A��a�ܷ���������ʴ��b�ܷ������ⸯʴ
B��һ��ʱ���a��Һ�����b��Һ��
C��a����Һ��pH����b����Һ��pH��С
D��a��b����������ͬ�ĵ缫��Ӧʽ��Fe��2e��===Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���A�������л�ũҩ��ɱ���顱��һ���м��壬��ṹ��ʽ����ͼ��������������ȷ���� ( )
A���л���A���ڷ�����
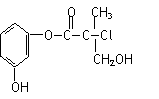 B���л���A�� Br2��CCl4��Һ�������ӳɷ�Ӧ
B���л���A�� Br2��CCl4��Һ�������ӳɷ�Ӧ
C���л���A��Ũ�����ϼ��ȣ����Է�����ȥ��Ӧ
D��1 mol A��������NaOH��Һ��Ӧ������������4 mol NaOH
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com