
����Ŀ��CH4����һ����Ҫ����Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϡ�
��1��������·ֽ�����������̼�����ܱ������н��д˷�ӦʱҪͨ����������ʹ���ּ���ȼ�գ���Ŀ����________��
��2����CH4Ϊȼ�Ͽ���Ƴɽṹ������ת���ʸߡ��Ի�������Ⱦ��ȼ�ϵ�أ��乤��ԭ����ͼ����ʾ����ͨ��a����ĵ缫����Ϊ_____��ͨ��b����ĵ缫��ӦʽΪ____�������ӽ���Ĥֻ����H+ͨ����
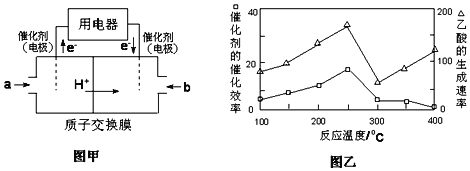
��3����һ���¶Ⱥʹ��������£�CH4��CO2��ֱ��ת�������ᣬ����ʵ������������һ���о�����
���ڲ�ͬ�¶��£������Ĵ�Ч�������������������ͼ����ʾ����÷�Ӧ������¶�Ӧ������__���ҡ�
�ڸ÷�Ӧ��������Ч�ɷ�Ϊƫ������ͭ��CuAlO2�����������CuAlO2�ܽ���ϡ���������������β��ų�NO���壬�����ӷ���ʽΪ___________��
��4��CH4��ԭ���Ǵ���NOx�����һ�ַ�������֪һ��������CH4��NOx���巴Ӧת��ΪN2��CO2������״����8.96LCH4�ɴ���22.4LNOx���壬��xֵΪ________��
���𰸡��ṩCH4�ֽ���������� ���� O2+4H++4e-=2H2O 250�� 3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO�� 1.6
��������
��1������ֽ���Ҫ������ȼ�տ��ṩ����������
��2����ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ���������������ˮ��
��3���ٸ������ᷴӦ��������������ѡ��
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���ƽ��д���ӷ���ʽ��
��4�����ݵ���ת���غ���㡣
(1)������·ֽ�����������̼�����ܱ������н��д˷�ӦʱҪͨ����������ʹ���ּ���ȼ�գ���Ŀ���ǣ��ṩCH4�ֽ�������������ʴ�Ϊ���ṩCH4�ֽ������������
(2)��ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ���������������ˮ�������缫��ӦʽΪ��O2+4H++4e-=2H2O���ʴ�Ϊ��������O2+4H++4e-=2H2O��
(3)��250��ʱ���ᷴӦ����������ԣ���ѡ��250�棬�ʴ�Ϊ��250�棻
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���Ӧ���ӷ���ʽΪ��3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO�����ʴ�Ϊ��3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO����
(4)���ݵ���ת���غ㣬��8.96L��[4(4)]=22.4L��2x�����x=1.6���ʴ�Ϊ��1.6��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ǿ�ѧѧϰ����Ҫ����֮һ������Ԫ�صĸ������ʿɹ�������Ϊ���±���ʾ�ı���(����)��
8O | 16S | 34Se | 52Te | |
�����۵�(��) | ��218.4 | 113 | 450 | |
���ʷе�(��) | ��183 | 444.6 | 685 | 989 |
��Ҫ���ϼ� | ��2 | ��2����4����6 | ��2����4����6 | |
ԭ�Ӱ뾶 | ������ | |||
������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
����ݱ��ش��������⣺
(1)�����۵㷶Χ������___________��
(2)�ڵĻ��ϼۿ�����__________��
(3)�������н�ǿ��________(������������������ԭ����)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)��ҵ��Al2Te3�������Ʊ�H2Te��������л�ѧ����ʽ��______Al2Te3��______=____Al(OH)3��______H2Te��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij���淴ӦaA(g)��bB(g)![]() cC(g)����H�����ܱ������н��С���ͼ��ʾ�ڲ�ͬʱ��t���¶�T��ѹǿp��B�����ڻ�������е����������(B)�ı仯����������ƶ�����ȷ����
cC(g)����H�����ܱ������н��С���ͼ��ʾ�ڲ�ͬʱ��t���¶�T��ѹǿp��B�����ڻ�������е����������(B)�ı仯����������ƶ�����ȷ����
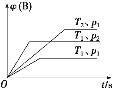
A. p1��p2��T1��T2��a��b��c����H��0
B. p1��p2��T1��T2��a��b��c����H��0
C. p1��p2��T1��T2��a��b��c����H��0
D. p1��p2��T1��T2��a��b��c����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ���̿�ʯ����Ҫ�ɷ�ΪMnO2��116g ������Ũ���ᷢ�����·�Ӧ(���ʲ����뷴Ӧ)MnO2+4HCl(Ũ) ![]() MnCl2+Cl2��+2H2O���Ƶ�22.4L Cl2����״�����������й�˵��������ȷ����
MnCl2+Cl2��+2H2O���Ƶ�22.4L Cl2����״�����������й�˵��������ȷ����
A���������̿�ʯ��MnO2����������Ϊ75%
B����������HCl�����ʵ���Ϊ4mol
C���μӷ�Ӧ��HCl������Ϊ146g
D������ԭ��MnO2�����ʵ���Ϊ1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧС��ѧ��������ͼ����װ�ý���������ˮ������Ӧ����ʵ�飬�����ò����һ����ȡFeCl36H2O���塣��ͼ�мгּ�β������װ�þ�����ȥ��

��1��װ��B�з�����Ӧ�Ļ�ѧ����ʽ��______��
��2��װ��E�е�������______��
��3��ֹͣ��Ӧ����B����ȴ��ȡ���еĹ��壬�������ϡ�����ַ�Ӧ�����ˡ�д�����ܷ������йط�Ӧ��ѧ����ʽ��______��
��4����С��ѧ������������Һ��ȡFeCl36H2O���壬���������ͼ��ʾ��
![]()
������I��ͨ��Cl2��������________��
������������Һ��Fe3+�IJ�������_______��
����������FeCl3ϡ��Һ�еõ�FeCl36H2O�������Ҫ����������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ե���Ϊ��Ҫԭ�Ϻϳ�һ�־��й���ζ������C�ͻ�����D�ĺϳ�·������ͼ��ʾ��

��ش��������⣺
��1�������ǵĽṹ��ʽΪ__________��B�����еĹ���������Ϊ_______��
��2����Ӧ��������X�ķ���ʽΪ_________����Ӧ�������Ϊ_________��
��3����Ӧ�۵Ļ�ѧ����ʽΪ_______��
��4����Ӧ�ݵĻ�ѧ����ʽΪ_________����Ӧ������ʵ��������ϩ��Ϊ��ȥ���п��ܻ��е�SO2Ӧѡ�õ��Լ���______��
��5����֪D����Է�����Ϊ118������̼������Ԫ�ص����������ֱ�Ϊ40.68%��5.08%������Ϊ��Ԫ�أ���D�ķ���ʽΪ_____��
��6�����鷴Ӧ�ٽ��г̶ȣ���Ҫ���Լ���________��
A ���Ƶ�Cu��OH��2����Һ B ��ˮ
C NaOH��Һ D FeCl3��Һ
��7����ϩ��ͬϵ���ϩ��CH2��CH��CH3������ͨ���Ӿ۷�Ӧ���ɸ߷��ӻ������ṹ��ʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Na2A��NaHA��Һ�ֱ����ϡ�ͣ���-lg c��Na+��=pNa��-lgc��OH-��=pOH������������Һ��pNa��pOH�Ĺ�ϵ������ͼ������˵����ȷ���ǣ� ��
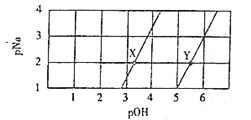
A. X�����ڵ�ֱ�߱�ʾNaHA��Һ��ϡ��
B. ��Y���������NaOH������ԴﵽX��
C. ��X��Y����Һ�������ϣ�����c��Na+��>c��A2-��>c��HA-��>c��OH-��>c��H+��
D. �����£�H2A��Ka1������Ϊ10-5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������⣨I2O5������������������ȥ�����е�һ����̼��������ˮ�γɵ��ᣬ��������ˮ�Ҵ������ѡ��ȷºͶ���̼��������װ���Ʊ����������⣨����װ�ü��г�װ��ʡ�ԣ���

�Ʊ�����������IJ������£�
����1��I2��KClO3��һ���������뷴Ӧ��M�У����������pH=1��2���¶ȿ�����80��90�棬���跴Ӧ1h��ʹ��Ӧ��ȫ��
����2��Ȼ����ȴ�����£�����������ؾ��塣�����˵õ��ľ����ˮ�������ܽ⣬������������������Һ�к�����ҺpHΪ10������ȴ�ᾧ�����˵õ��ľ�����118�����3h���õ�����ز�Ʒ��
����3��������2�Ƶõĵ�����ữ��õ��ᣨHIO3�����ٽ������ڸ�����������м��ȵ�200��ʧˮ�õ����������⡣
��1������������ɳ�ȥ�����е�һ����̼����Ӧ���ɵⵥ�ʣ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ___��
��2������M��������___��
��3������1�г������ɵ������[KH��IO3��2]�⣬ͬʱ�������Ȼ��غ���������n��KCl����n��C12��=5��3��д���÷�Ӧ�Ļ�ѧ����ʽ��___���÷�Ӧ�е���������___���ѧʽ����
��4�����۵�©��N���˿��Է�ֹ����������һ��������____��NaOH��Һ��������___��
��5������2�е���Ҫ��ӦΪKH��IO3��2+KOH===2KIO3+H2O����ȡ0.550g����ز�Ʒ���������ʲ����뷴Ӧ��������Ʒ�����ձ��У�������ˮ�ܽ⣬�����������ữ��KI��Һ�����Ƴ�100mL��Һ��ȡ10.00mL���Ƶ���Һ����ƿ�в�����ָʾ����Ȼ����0.1mol��L-1 Na2S2O3����Һ�ζ����յ㣬����ʵ��ƽ�����ı���Һ�����Ϊ15.00mL������֪I2+2Na2S2O3=2NaI+Na2S4O6��
�ٸõζ�ѡ���ָʾ����_____��
�ڸõ���ز�Ʒ�е���ص�����������____%��������λ��Ч���֣���
����װNa2S2O3����Һ�ĵζ���û����Na2S2O3����Һ��ϴ��������ò�Ʒ����������___���ƫ�ߡ���ƫ�͡�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ء�����˵���������
A. PM2.5��ָ����������2.5��m�Ŀ���������������
B. ��ɫ��ѧҪ���Դͷ�����������������Ի�������Ⱦ
C. ȼú�м���CaO���Լ���������γɼ�����������ŷ�
D. ��Ȼ����Һ��ʯ�������ҹ�Ŀǰ�ƹ�ʹ�õ����ȼ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com