

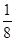 ��12��
��12�� ��6��
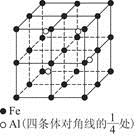
��6��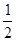 ��1��8������ԭ��ȫ��λ�ھ����ڣ�Ϊ4�������仯ѧʽ��д��Fe2Al������Ͻ������������Feԭ��֮��ľ���Ϊa�����ı߳�Ϊ2a�������Ϊ(2a)3��8a3��
��1��8������ԭ��ȫ��λ�ھ����ڣ�Ϊ4�������仯ѧʽ��д��Fe2Al������Ͻ������������Feԭ��֮��ľ���Ϊa�����ı߳�Ϊ2a�������Ϊ(2a)3��8a3�� ����
����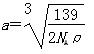 ��
��

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/molI2=1451kJ/molI3=7733kJ/molI4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
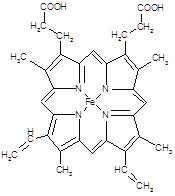
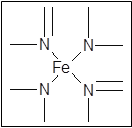
 ����___________mol��
����___________mol��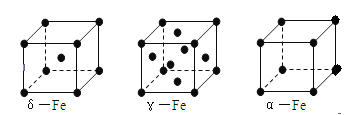
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
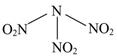
| A��������N��O���γɵĹ��ۼ��ǷǼ��Լ� | B���������ĸ���ԭ�ӹ�ƽ�� |
| C�������ʼ������������л�ԭ�� | D��15.2 g�����ʺ���6.02��1022��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
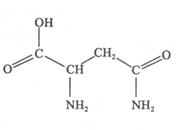
| ��ѧʽ | ������nm | ���� | �е㣯�� |
| H2S | 1.34 | 92.3o | һ60.75 |
| H2Se | 1.47 | 91.0o | һ41.50 |
| ��� | I5��kJ��mol-1 | I6��kJ��mol-1 | I7��kJ��mol-1 | I8��kJ��mol-1 |
| A | 6990 | 9220 | 11500 | 18770 |
| B | 6702 | 8745 | 15455 | 17820 |
| C | 5257 | 6641 | 12125 | 13860 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
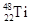 ��
�� ����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
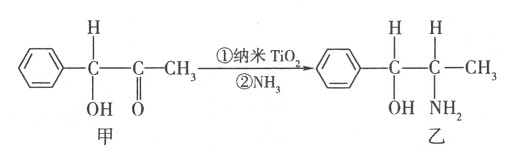
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com