
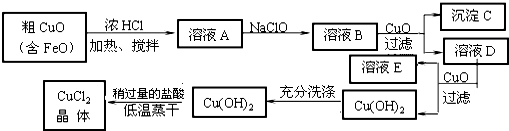
��10�֣���ҵ����ȡCuCl2�������������£�
�����±����ݣ��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� �� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� �� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 | �ܶȻ�/25�� | 8.0��10-16 | 2.2��10-20 | 4.0��10-38 | ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������Ƕ��и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��10�֣���ҵ����ȡCuCl2�������������£�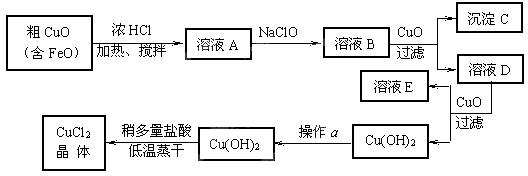
�����±����ݣ��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�����и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��10�֣���ҵ����ȡCuCl2�������������£�

�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��[��Դ:]
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ĸ߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��8��,ÿ��2�֣���ҵ����ȡCuCl2�������������£�
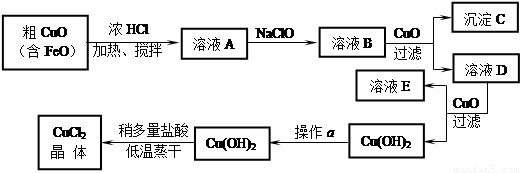
�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
�ܶȻ���25�� |
8.0��10��16 |
2.2��10��20 |
4.0��10��38 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com