
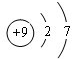 ��C2-�ĵ���ʽ
��C2-�ĵ���ʽ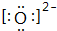 ��
�� ��
������ �к˵������1-18֮�������Ԫ��A��B��C��D��E�����ǵĺ˵����������������A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ����У�C�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪOԪ�أ�B��C��D���Ӳ�����ͬ��B���������4�����ӣ���BΪ̼Ԫ�أ�D��ԭ��������������D��E�γ����ӻ���������ӻ����������ǻ��ϼ۵ľ���ֵ��ȣ���DΪFԪ�ء�EΪNa��A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ���AΪHԪ�أ��ݴ˽��
��� �⣺�к˵������1-18֮�������Ԫ��A��B��C��D��E�����ǵĺ˵����������������A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ����У�C�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪOԪ�أ�B��C��D���Ӳ�����ͬ��B���������4�����ӣ���BΪ̼Ԫ�أ�D��ԭ��������������D��E�γ����ӻ���������ӻ����������ǻ��ϼ۵ľ���ֵ��ȣ���DΪFԪ�ء�EΪNa��A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ���AΪHԪ�أ�
��1��A������Ϊ�⣬DΪFԪ�أ�ԭ�ӵĽṹʾ��ͼΪ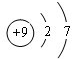 ��O2-�ĵ���ʽΪ
��O2-�ĵ���ʽΪ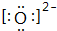 ��
��
�ʴ�Ϊ���⣻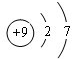 ��
��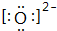 ��
��
��2��D��E�γɵ����ӻ�����ΪNaF�����뷽��ʽΪNaF=Na++F-��
�ʴ�Ϊ��NaF=Na++F-��
��3��B��C�м���NԪ�أ��䵥�ʵĽṹʽΪN��N��
�ʴ�Ϊ��N��N��N��
��4��E����������H2O��Ӧ�����������ƣ�����ʽΪ ��
��
�ʴ�Ϊ���������ƣ� ��
��
��5��A��B�γɵ�ij��̬�������������Ȼ������Ҫ�ɷ֣���֪1g������ȼ������CO2�����Һ̬H2Oʱ�ų�55.6kJ����������1mol������ȫȼ�շų�������Ϊ55.6kJ��$\frac{1mol��16g/mol}{1g}$=889.6kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã����ضԻ�ѧ����Ŀ��飬�ƶ�Ԫ���ǽ���ؼ���ע��Ի���֪ʶ���������գ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
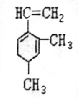 ��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������
��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������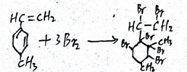 ��
��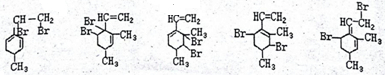 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4��Һ | B�� | BaCl2��Һ | C�� | NaOH��Һ | D�� | AgNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥFe2O3��Al2O3������е�Fe2O3��NaOH��Һ | |
| B�� | ��ȥNa2O2�е�Na2O������ | |
| C�� | ��ȥNa2CO3�����е�NaHCO3����NaOH���� | |
| D�� | ��ȥFe��C�Ͻ��е�C����O2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��a=d��b | B�� | b��a=d��c | C�� | c��a��b��d | D�� | a=b��c��d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ | B�� | AgNO3��Һ | C�� | BaCl2��Һ | D�� | Ba��OH��2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
��Դ�ɷ�Ϊһ����Դ�Ͷ�����Դ����Ȼ�������ֳ���ʽ�ṩ����Դ��Ϊһ����Դ��������������Դ�����ȡ����Դ��Ϊ������Դ��������������һ�ָ�Ч��û����Ⱦ�Ķ�����Դ���ݴ��жϣ�����������ȷ����( )
A����Ȼ���Ƕ�����Դ B��ʯ���Ƕ�����Դ
C����¯ú����һ����Դ D��ú��һ����Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com