
| �� ���� |
I A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H |
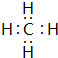 ���ʴ�Ϊ��
���ʴ�Ϊ��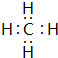 ��
�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ں����ܱ�������ͨ��X��������Ӧ��2X��g��?Y��g�����¶�T1��T2��X�����ʵ���Ũ��c��X����ʱ��t�仯��������ͼ��ʾ������������ȷ���ǣ�������
�ں����ܱ�������ͨ��X��������Ӧ��2X��g��?Y��g�����¶�T1��T2��X�����ʵ���Ũ��c��X����ʱ��t�仯��������ͼ��ʾ������������ȷ���ǣ�������| A���÷�Ӧ���е�M��ų����������ڽ��е�W��ų������� | ||
B��T2�£���0��t1ʱ���ڣ�v��Y��=
| ||
| C��M�������Ӧ����V������N����淴Ӧ����V�� | ||
| D��M��ʱ�ټ���һ����X��ƽ���X��ת���ʼ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
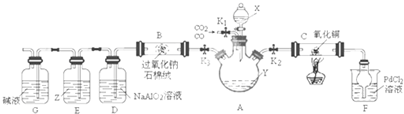
| ��� | ʵ����� | ʵ����������� | �� �� | ||
| �� | ����a g M�м���һ����ϡ���ᣬ��ֽ��裻 �ڼ����μ�ϡ��������������ַ�Ӧ�� |
�ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ |
��M��һ����Cu2O�� ��M��һ����Cu�� | ||
| �� | �ٽ���ʵ���������Һ���� �ڽ�����ϴ�ӡ�������� |
��������Ϊ
|
MΪCu��Cu2O�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
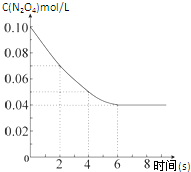 ���¶�Ϊ298Kʱ����0.10mol��ɫ��N2O4�������1L��յ��ܱ������У����ֺ���ɫ��ֱ������N2O4��g��?2NO2��g����ƽ�⣮��ͼ��ʾ�ⶨN2O4��Ũ����ʱ���ϵ�����ߣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩��
���¶�Ϊ298Kʱ����0.10mol��ɫ��N2O4�������1L��յ��ܱ������У����ֺ���ɫ��ֱ������N2O4��g��?2NO2��g����ƽ�⣮��ͼ��ʾ�ⶨN2O4��Ũ����ʱ���ϵ�����ߣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩��| T/K | 310 | 320 |
| Kֵ | 0.38 | 0.42 |
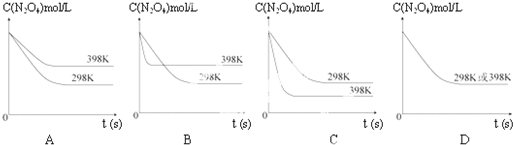
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��2 | B��1��3 |
| C��1��6 | D��3��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com