
ij��ѧ����С�����ʵ��̽�����Ļ���������ʣ�װ������ͼ��ʾ��Aװ��δ������������AΪ���巢��װ�á�A�������Լ��������й���������ѡȡ��
a. NH4HCO3 b. NH4Cl c. Ca(OH)2 d. NaOH
![]()
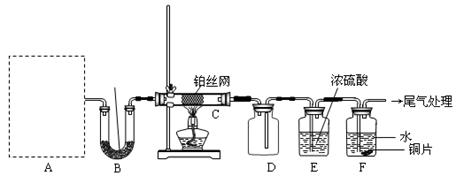
���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ �̺�ȥC���ƾ��ơ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⡣
��1��ʵ������ȡA������ʱ��ֻ��һ���Լ������Լ��� ���������ѡ�Լ�����ĸ������ʱA����Ҫ�IJ��������� �������ƣ���
��2��A�в��������ʱ�B��Na2O2������գ�д������һ��B�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3������C�з����Ŀ��淴Ӧ������˵����ȷ�� ��
a. ����һ�ַ�Ӧ���Ũ�ȿ��������һ�ַ�Ӧ���ת����
b. ��ҵ�Ͻ��и÷�Ӧʱ���ɲ�ȡ��ѹ��������߷�Ӧ��ת����
c. �÷�Ӧ��һ�������´ﵽƽ��ʱ����Ӧ���ƽ��Ũ��֮��һ����4:5
d. ʹ�ò�˿������ʹ����Ӧ��������ͬʱҲ�����淴Ӧ����
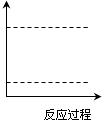
��4������ͼ�����л���Cװ���з�Ӧ���������е������仯ʾ��ͼ������������ֵ���ñ���������������Ϸֱ�����Ӧ���������Ļ�ѧʽ��
��5����ʵ�������B�й�����������500 mL 1 mol��L��1 �������У�������ɫ�������ף���Һ�����ԣ���ʵ��ǰB��ԭ��Na2O2�����ʵ����� mol�����ڱ�״�������Ϊ L�����������ܽ⣩��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��֪ʶ����������ѵ������������ѧ ���ͣ�013
ij��ѧ����С��Ϊ�ⶨij��������Һ��ʯ����(���Һ��ʯ�����ɶ���ͱ������)��ƽ����Է�����������ʵ�������ռ��û��������Ʒ�������ռ���������ȷ����
[����]


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�013

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
ij��ѧ����С��Ϊ�ⶨij��������Һ��ʯ���������Һ��ʯ�����ɶ���ͱ�����ɣ���ƽ����Է�����������ʵ�������ռ��û��������Ʒ�������ռ�������ȷ������������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058

��1��д���������ʵ����ƣ�A________��B________��F________��
��2��I�и������ʹ�ã����ţ�________��
����ʯ������
����ʯ��
����ˮCaCl2 ��ŨH2SO4
��3��д�����л�ѧ����ʽ�������ӷ�Ӧ��ֻд���ӷ���ʽ��
��A��B�����壺______________________________________________________��
��F��ͨ�������壺____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д���������ʵ����ƣ�
A.__________________��B.__________________��F.__________________��
(2)д�����л�ѧ����ʽ�������ӷ�Ӧ��ֻд���ӷ���ʽ��
��A+B�����壺___________________________��
������+F��___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com