
�±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
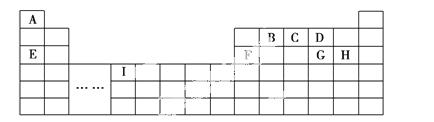
(1)д������������Ԫ�����X2Y2�ͻ�����Ļ�ѧʽ�ֱ�Ϊ____________���侧�����ͷֱ�Ϊ__________________��
(2)B��C��D���⻯���У��е���ߵ��⻯��Ľṹʽ��____________�������е���͵��⻯��Ŀ����������____________��
(3)����Ԫ���γɵĵ����У�����ԭ�Ӿ������________(������)����ռ乹��Ϊ________________��
(4)д��������Ԫ������ͬ������Ԫ����ɵ����ֻ������ˮ��Һ������Ӧ�����ӷ���ʽ��_____________________��
(5)д��������Ԫ���е������γɵļȺ������Ӽ��ֺ��м��Թ��ۼ��Ļ�����ĵ���ʽ��____________________(��д����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ�������ʾ��ͼ���£�

�ش��������⣺
(1)��ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽����________��________����������Դ��ˮ��̼�⻯���д���ֱ����ú����Ȼ��Ϊԭ����ȡ�����Ļ�ѧ����ʽ_______________________��
(2)�豸A�к��е����������ý���Ƚ��������豸A��������________�����з����Ļ�ѧ��Ӧ����ʽΪ____________��
(3)�豸B������Ϊ________������m��n������ͨˮ�ڣ���ˮ����________(�m����n��)�����˴��෴����ͨˮ��ԭ����______________��
(4)�豸C��������______________________��
(5)��ԭ�����Ʊ������л��е�CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�
CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��֪1 000 Kʱ�÷�Ӧ��ƽ�ⳣ��K��0.627����ҪʹCO��ת���ʳ���90%������ʼ����c(H2O)��c(CO)������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ�������·���˺��ձ���ѧ�Ҹ���Ӣһ����ľ�������з����л��ϳ��е��ٴ��Ľ���ż���������2010���ŵ������ѧ�����л��ϳɳ��õ���/����̿����������ʹ�ô����ᱻ����(�磺�����л����)��Ⱦ��ʧȥ���ԣ���Ϊ�ϴ�����������������ա�һ���ɷϴ�����ȡ�Ȼ��ٵĹ����������£�
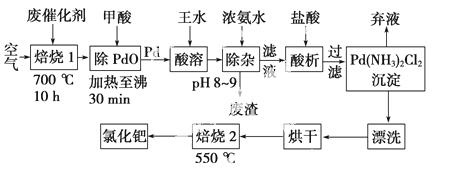
(1)���ٴ�������ɺ�����700 ��ĸ����±��գ����չ�������ͨ������������ԭ����________________________________________________________________________��
���ỹԭ�����ٵĻ�ѧ����ʽΪ____________________ ______________________��
______________________��
(2)������ˮ(Ũ������Ũ���ᰴ�����1��3)��ת��ΪH2PdCl4�����ỹԭΪNO���÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
(3)�پ������ٵĻ����ʸߵ���Ҫȡ������ˮ�ܽ�IJ�����������֪��Ӧ�¶ȡ���Ӧʱ�����ˮ�������ٻ����ʵ�Ӱ������ͼ1��ͼ3��ʾ������ˮ�ܽ��پ�������������(�¶ȡ�ʱ�����ˮ����)Ϊ__________��__________��__________��

(4)��Ũ��ˮʱ����ת��Ϊ������[Pd(NH3)2]2������ʱ���Ĵ�����ʽ��________________________________________________________________________(д��ѧʽ)��
(5)700 �決��1��Ŀ����________________��550 �決��2��Ŀ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڷ� �ֵ�һ����Ȼ������������Al��Cu��FeԪ�����
�ֵ�һ����Ȼ������������Al��Cu��FeԪ����� ���ش��������⣺
���ش��������⣺
��1������һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ�� �������־��塢����ͷǾ��塣
��2����̬��ԭ���� ��δ�ɶԵ��ӣ����������ӵĵ����Ų�ʽΪ�� �������軯�ؼ������������ӣ��γ���������ɫΪ
��3�����Ʊ���������ͭ�ɽ���ȩ����Ϊ���ᣬ��������ԭ������ ��ͭ����ȩ��̼ԭ�ӵ��ӻ��������Ϊ ______��һĦ����ȩ�����к��еĦҼ�����ĿΪ�� ������ķе����Ը�����ȩ������Ҫԭ���ǣ� ��������ͭΪ�뵼����ϣ��������������ڲ����ĸ���ԭ�ӣ�������ԭ��λ�����ĺͶ��㣬��þ������� ��ͭԭ�ӡ�
��ͭ����ȩ��̼ԭ�ӵ��ӻ��������Ϊ ______��һĦ����ȩ�����к��еĦҼ�����ĿΪ�� ������ķе����Ը�����ȩ������Ҫԭ���ǣ� ��������ͭΪ�뵼����ϣ��������������ڲ����ĸ���ԭ�ӣ�������ԭ��λ�����ĺͶ��㣬��þ������� ��ͭԭ�ӡ�
��4��������Ϊ�����������壬�侧������a��0��405nm����������ԭ�ӵ���λ��Ϊ ����ʽ��ʾ�����ʵ��ܶ� g·cm��3(���ؼ�������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ��,���ǵĺ˵������������Aԭ��L��ijɶԵ�������δ�ɶԵ��������,Bԭ�ӵ������p����ĵ���Ϊ������ṹ,C�ǵؿ��к�������Ԫ�ء�D�ǵ�������Ԫ��,��ԭ�Ӻ�����������������ԭ����ͬ,���������Ӿ���������ش���������:
(1)A��B��C�ĵ�һ��������С�����˳������������(�ö�Ӧ��Ԫ�ط��ű�ʾ);��̬Dԭ�ӵĵ����Ų�ʽΪ������������������
(2)A������������Ӧ��ˮ���������,������ԭ�Ӳ�ȡ���������ӻ�;B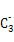 �Ŀռ乹��Ϊ��������(����������)��
�Ŀռ乹��Ϊ��������(����������)��
(3)1 mol AB-�к��еĦм�����Ϊ����������
(4)��ͼ�ǽ���Ca��D���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ,��úϽ���Ca��D��ԭ�Ӹ�����������������
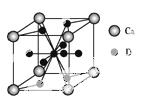
(5)�����Ͻ��������Ͻ�����ͬ���͵ľ����ṹXYn,�����к�ǿ�Ĵ�����������֪�����Ͻ�LaNin�������Ϊ9.0��10- 23 cm3,������γ�LaNinH4.5�Ͻ�(����뾧����϶,�������),��LaNin��n=��������(����ֵ);���ںϽ��е��ܶ�Ϊ����������
23 cm3,������γ�LaNinH4.5�Ͻ�(����뾧����϶,�������),��LaNin��n=��������(����ֵ);���ںϽ��е��ܶ�Ϊ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1)��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ________��
��NO �Ŀռ乹����________(����������)��
�Ŀռ乹����________(����������)��
(2)��ͭ��������Ĵ��£�CO������ΪCO2��HCHO������ΪCO2��H2O��
�ٸ��ݵȵ�����ԭ����CO���ӵĽṹʽΪ________��
��H2O������Oԭ�ӹ�����ӻ�����Ϊ________��
��1 mol CO2�к��еĦҼ���ĿΪ________��
(3)��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڻ�ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ����a�� Ni2+ + H2PO2��+ ��  Ni++ H2PO3��+
Ni++ H2PO3��+
���������д������ƽ��Ӧʽ��a����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���жԡ�Ħ�� mol������������ȷ��������
A��Ħ����һ����λ�����ڼ����������������ӵĶ���
B��Ħ������������������������������������
C��1 mol�κ��������������������Ŀ�����
D���á�Ħ���������á������������������á����ס������á��ס�����ԭ��ֱ��������˼·��������λ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����(����)��
A����״���£�33.6 L�������к��з�ԭ�ӵ���ĿΪ1.5NA
B�����³�ѹ�£�7.0 g��ϩ���ϩ�Ļ�����к�����ԭ�ӵ���ĿΪNA
C��50 mL 18.4 mol·L-1Ũ����������ͭ�ȷ�Ӧ������SO2���ӵ���ĿΪ0.46NA
D��ij�ܱ�����ʢ��0.1 mol N2��0.3 mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0.6NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com