
FeSO4��7H2O�㷺����ҽҩ��ҵ����
��1���������Թ�ҵ����мΪԭ������FeSO4��7H2O������ͼ��

����д���пհס�
�ټ�����̼������Һ��Ŀ����______________����ӦI��Ҫ���������ӣ���ԭ����___________��
���жϷ�Ӧ����ɵ�������__________����Ӧ����Ҫ100mL1mol��L��ϡ���ᣬ��98��3�����ѣ�1��84g��cm3��Ũ�������ơ����õ���������Ͳ���ձ�������������ͷ�ιܼ�____________.
�۲ⶨFeSO4��7H2O��Ʒ��Fe2�������ķ�������KMnO4��Һ�ζ�����5Fe2���� ��8H����5Fe3����Mn2����4H2O��������Ϊ��
��8H����5Fe3����Mn2����4H2O����������
��ȡ2��8500g FeSO4��7H2O��Ʒ�����Ƴ�250mL��Һ��
����ȡ25��00mL������Һ����ƿ�У�
���������ữ��0��01000moL��L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20��00mL��

ijͬѧ�����ͼ��ʾ�ĵζ���ʽ�У����������____________���гֲ�����ȥ��������ĸ��ţ����жϴ˵ζ�ʵ��ﵽ�յ�ķ�����____________������������Ʒ��FeSO4��7H2O����������Ϊ________����С����ʾ��������λС�������������������ⶨ����Ʒ��FeSO4��7H2O����������ƫ�ͣ��ⶨ�����в��������ɺ��ԣ��������ԭ����_____________��______________��
��2����֪FeSO4��7H2O�����ڼ��������·������·�Ӧ��2FeSO4��7H2O Fe2O3��SO2����SO3����14H2O����������ͼװ�ÿɼ���÷�Ӧ��������
Fe2O3��SO2����SO3����14H2O����������ͼװ�ÿɼ���÷�Ӧ��������

����д���пհס�
������������˳��Ϊa��________��__________��_________��________��__________��__________��_____________��
��װ��C�е�XΪ______________����װ������ˮ��������_________________��
��15�֣���1���ٳ����ۣ�1�֣� ���´ٽ�ˮ�⣬��Һ������ǿ��ȥ����������ǿ��1�֣�
�ڹ��岻���ܽ⣬������治�������ݲ�����1�֣�
100ml����ƿ��2�֣���ֻ������ƿ1�֣�
��b��1�֣� �μ����һ��KMnO4��Һʱ����Һ���dz��ɫ�Ұ�����ڲ���ɫ��1�֣�
0.975��2�֣� ��Ʒ�д������������ʣ�1�֣� ��Ʒ���ֱ�������1�֣�
��2����f��g��d��e��h��i��b����g��f��d��e��h��i��b����2�֣�
��BaCl2��1�֣� SO3��H2O�ķ�Ӧ�Ƿ��ȷ�Ӧ�������¶�������SO3��ˮ���գ�1�֣�
��������
�����������1�������ڹ�ҵ����м�к����������ۣ����Ա����ȥ�������ۡ�̼������Һ�Լ��ԣ������ܽ��������۴ﵽȥ����Ŀ�ġ����������¶ȴٽ�ˮ�⣬��Һ������ǿ��ȥ����������ǿ�����Է�ӦI��Ҫ���������ӡ�
�ڷ�Ӧ��������ϡ����ķ�Ӧ�������������������������������жϷ�Ӧ����ɵ������ǹ��岻���ܽ⣬������治�������ݲ���������һ�����ʵ���Ũ����Һʱ�����õ�������������Ͳ���ձ�������������ͷ�ι����⣬��������100ml����ƿ��
�����Ը��������ҺӦ�÷�����ʽ�ζ����У�a�����Ը��������Һ���ڼ�ʽ�ζ����У����a����ȷ��b��ȷ������������Һ�����ԣ�Ӧ�÷�����ʽ�ζ����У�ѡ��c����ȷ����ѡb���������Ը��������Һ�Ժ�ɫ�����Ը�ʵ�鲻��Ҫָʾ��������жϴ˵ζ�ʵ��ﵽ�յ�ķ����ǵμ����һ��KMnO4��Һʱ����Һ���dz��ɫ�Ұ�����ڲ���ɫ�����ݷ���ʽ��֪
5FeSO4��7H2O��KMnO4
5mol 1mol
n 0.01000moL��L��0.0200L
���n��0.0010mol
����������Ʒ��FeSO4��7H2O����������Ϊ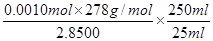 ��0.975��
��0.975��
���������������ױ������������������������ⶨ����Ʒ��FeSO4��7H2O����������ƫ�ͣ���˿��ܵ�ԭ������Ʒ���ֱ���������Ʒ�д������������ʡ�
��2���ټ���ˮ����һ������ˮ����ͭ������SO2һ����Ʒ����Һ������SO2���۷е�ϵͣ����SO3�����ױ�ˮ�����������ᣬ����ͨ����������������SO3������Ϊͨ����Һʱ��Ȼ����ˮ�������������ȼ���ˮ��������μ���SO3��������SO2���һ�Ҫ��β������װ�ã������ȷ��˳����a��f��g��d��e��h��i��b����a��g��f��d��e��h��i��b����
�ڼ���������Լ��������Ȼ�����Һ������ΪSO3��H2O�ķ�Ӧ�Ƿ��ȷ�Ӧ������װ������ˮ�������ǽ����¶ȣ�����SO3��ˮ���ա�
���㣺�����������������Ʊ���ʵ��̽�������ʵ���Ũ����Һ�����ơ���������Է�Ӧ���ʵ�Ӱ�졢�ζ�ʵ����жϡ����ʺ����IJⶨ�Լ������������ʵļ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ����� ʵ������ |
1 | 2 | 3 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 |
| V��KMnO4��/mL���������� | 0.00 | 0.20 | 0.00 |
| V��KMnO4��/mL���ն����� | 15.85 | 15.22 | 14.98 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H+ |
| ��ת�� |
| Fe2+ |
| �ڻ�ԭ |
| OH- |
| �۳��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H+ |
| ��ת�� |
| Fe2+ |
| �ڻ�ԭ |
| OH- |
| �۳��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com