
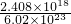 mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��
mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��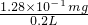 =0.64mg/L�������ϴ�������������
=0.64mg/L�������ϴ������������� Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O�� Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�043
�ҹ�ũҵ��������������ÿ����ʧ�ߴ�15����Ԫ��Ϊ����Ч�������꣬Ŀǰ����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��森
(1)����1����ˮ��Ʒ��ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�

�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ����(�û�ѧ����ʽ��ʾ)_______________��
���������ȡ����������ˮ������ˮ���ϣ�pH����_______________��ԭ����(�û�ѧ����ʽ��ʾ)_______________��
(2)����Ϊ�������������;���ɲ��õĴ�ʩ��
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
[����]
(3)��Ӣ�����е�һ���о�������������̴ѿ�����Ч�ؽ��͵ر��� Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų���
Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų��� Ũ�Ƚ�����35���������ڽ������̴ѵĽ��������
Ũ�Ƚ�����35���������ڽ������̴ѵĽ�������� Ũ�Ƚ�����30��֮�࣮�����ȫ�������ĽǶȣ��������ַ����Ƿ��ȡ�����������ɣ�
Ũ�Ƚ�����30��֮�࣮�����ȫ�������ĽǶȣ��������ַ����Ƿ��ȡ�����������ɣ�
(4)�ô�ͳ��ú��ʯ����ȼ�ϣ�����Ҫȱ����ʲô���봫ͳ��ú��ʯ��ȼ����ȣ��������ʿ���Ϊ�µ���Դ����Ҫ�ŵ�����ʲô��
(5)Ϊ�˷������꣬����úȼ��ʱ������ŷ� ����ҵ�ϳ�����ʯ�Һͺ���ú��Ϻ�ʹ�ã���д��ȼ��ʱ���йء�����(��ʹ����������)��Ӧ�Ļ�ѧ����ʽ��_______________�����Ƚϴ˷��뽫��ʯ��ʯ��ĩ�뺬��ú��ϡ���ɷ�������ķ������ĸ�����Щ��_____________(���ʯ�ҡ���ʯ��ʯ����)��
����ҵ�ϳ�����ʯ�Һͺ���ú��Ϻ�ʹ�ã���д��ȼ��ʱ���йء�����(��ʹ����������)��Ӧ�Ļ�ѧ����ʽ��_______________�����Ƚϴ˷��뽫��ʯ��ʯ��ĩ�뺬��ú��ϡ���ɷ�������ķ������ĸ�����Щ��_____________(���ʯ�ҡ���ʯ��ʯ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�������и������£��ھŴ��¿���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com