
|
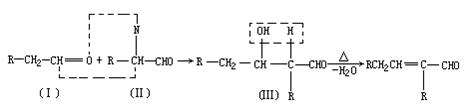
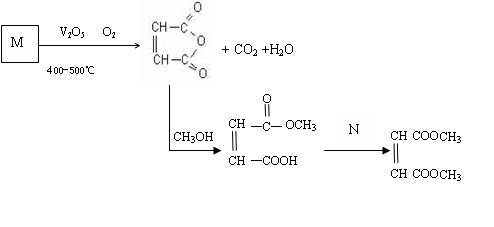 £Ø1£©ŅŃÖŖĢžMµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ78£¬·Ö×ÓÄŚŗ¬Ģ¼ĮæĪŖ92.3£„£¬ĖłÓŠC”ŖH¼üŗĶĢ¼Ģ¼¼üĄąŠĶĻąĶ¬£¬»ÆŗĻĪļMµÄ»ÆѧŹ½ĪŖ
£Ø1£©ŅŃÖŖĢžMµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ78£¬·Ö×ÓÄŚŗ¬Ģ¼ĮæĪŖ92.3£„£¬ĖłÓŠC”ŖH¼üŗĶĢ¼Ģ¼¼üĄąŠĶĻąĶ¬£¬»ÆŗĻĪļMµÄ»ÆѧŹ½ĪŖ 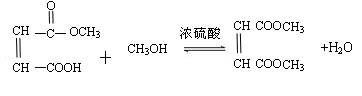
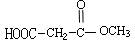 £»
£»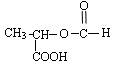
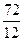 =6£¬Č»ŗóĶØŹ½CxHyĒó³öĒāŌ×ÓøöŹżĪŖ6£¬MµÄ·Ö×ÓŹ½ĪŖC6H6£»£Ø3£©ŅĄ¾ŻĢāŅāæÉÖŖ£¬“ÖĘ·ŗĶ“æĘ·µÄĒų±šŌŚÓŚ“ÖĘ·ÖŠŗ¬ÓŠōČ»ł£¬¶ųĖ³¶”Ļ©¶žĖį¶ž¼×õ„»Æѧ“æÖŠÖ»ÓŠõ„»ł£¬ĖłŅŌæÉŅŌĄūÓĆōČ»łÓėNaHCO3·“Ó¦²śÉśĘųĢåĄ“¼ģŃ飻£Ø4£©NĪŖ¼×“¼£¬¼“ĪŖ¼×“¼ÓėÖŠ¼ä²śĪļA·¢Éśõ„»Æ·“Ó¦£»£Ø5£©
=6£¬Č»ŗóĶØŹ½CxHyĒó³öĒāŌ×ÓøöŹżĪŖ6£¬MµÄ·Ö×ÓŹ½ĪŖC6H6£»£Ø3£©ŅĄ¾ŻĢāŅāæÉÖŖ£¬“ÖĘ·ŗĶ“æĘ·µÄĒų±šŌŚÓŚ“ÖĘ·ÖŠŗ¬ÓŠōČ»ł£¬¶ųĖ³¶”Ļ©¶žĖį¶ž¼×õ„»Æѧ“æÖŠÖ»ÓŠõ„»ł£¬ĖłŅŌæÉŅŌĄūÓĆōČ»łÓėNaHCO3·“Ó¦²śÉśĘųĢåĄ“¼ģŃ飻£Ø4£©NĪŖ¼×“¼£¬¼“ĪŖ¼×“¼ÓėÖŠ¼ä²śĪļA·¢Éśõ„»Æ·“Ó¦£»£Ø5£©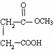 £¬õ„Ąą½į¹¹æÉÄÜÓŠ£ŗHCOOCH2CH2COOCH3”¢CH3COOCH2COOCH3”¢CH3CH2COOCOOCH3”¢HCOOCH2COOCH2CH3”¢CH3COOCOOCH2CH3µČ”£
£¬õ„Ąą½į¹¹æÉÄÜÓŠ£ŗHCOOCH2CH2COOCH3”¢CH3COOCH2COOCH3”¢CH3CH2COOCOOCH3”¢HCOOCH2COOCH2CH3”¢CH3COOCOOCH2CH3µČ”£

 ĆūŠ£ĶØŠŠÖ¤ÓŠŠ§×÷ŅµĻµĮŠ“š°ø
ĆūŠ£ĶØŠŠÖ¤ÓŠŠ§×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
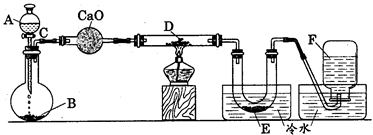 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
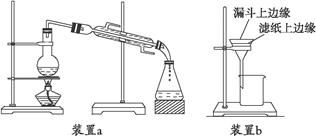
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
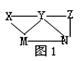
| A£®XŅ»¶ØĪŖH2SO4 |
| B£®YŅ»¶ØĪŖK2CO3 |
| C£®ZæÉÄÜĪŖĀČĖ® |
| D£®M”¢N±Ų¶Øø÷ĪŖBaCl2”¢FeSO4ÖŠµÄŅ»ÖÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
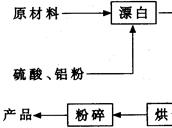
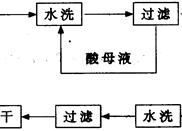
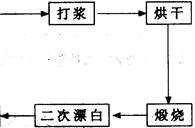
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
|

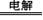 (NH4)2S2O8 + H2”ü£¬
(NH4)2S2O8 + H2”ü£¬
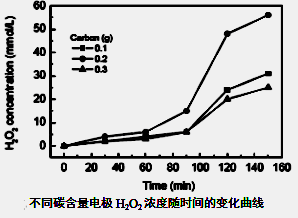
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
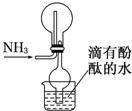
| A£®ŹÕ¼Æ°±ĘųµÄŌĄķŹĒĻņĻĀÅÅæÕĘų·Ø |
| B£®øÉŌļ¹ÜµÄ×÷ÓĆŹĒ·ĄÖ¹µ¹Īü |
| C£®µ±ÉÕ±ÖŠµÄĖ®±äŗģŹ±Ö¤Ć÷°±ĘųŅŃŹÕ¼ÆĀś |
| D£®øĆ×°ÖĆ»įŠĪ³ÉŗģÉ«ÅēČŖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¹żĮæµÄĢśÓėĻ”ĮņĖį·“Ó¦ |
| B£®FeOÓėĻ”H2SO4·“Ó¦ |
| C£®FeCO3ÓėĻ”H2SO4·“Ó¦ |
| D£®Fe(OH)2ÓėĻ”H2SO4·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com