
| n |
| V |
| 0.4mol/L��0.25L |
| 18.4mol/L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| A���٢ۢ� | B���٢ۢޢ� |
| C���ۢܢޢ� | D���ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�����ڼ��л���������Ʊ������롢�ᴿ�����ļ���װ�ã�����ݸ�װ�ûش��������⣺
��ͼ�����ڼ��л���������Ʊ������롢�ᴿ�����ļ���װ�ã�����ݸ�װ�ûش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
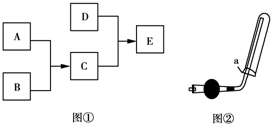 ��֪A��B��C��DΪ���壬����AΪ����ɫ��D��������ˮ���γɵ���Һ��ʹ��̪��죬����֮���ת����ϵ��ͼ����ʾ��
��֪A��B��C��DΪ���壬����AΪ����ɫ��D��������ˮ���γɵ���Һ��ʹ��̪��죬����֮���ת����ϵ��ͼ����ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� |
IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HCl��Na2CO3 HCl��NaHCO3 |
| B��AgNO3��HCl AgNO3��H2SO4 |
| C��BaCl2��Na2SO4 Ba��OH��2��CuSO4 |
| D��KOH��CuCl2 Ba��OH��2��CuCl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com