
A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ�������¢�B��Һ�ֱ���C��D��ϣ����а�ɫ�������ɢڽ�A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С_��___��___��___�������ӷ��ţ���B��������������____��____
��2��C��Һ��__��_�ԣ�����ԡ����ԡ�������ԭ����______��____________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��_____��_______
��3�� ��PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ___��____�����������Һ������䣩�������ĵ缫��ӦʽΪ��___��__
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ____________��__________ ____
____

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������п����2mol/L������Һ��ȡ���������д�ʩ������ѧ��Ӧ���ʵ��ǣ���
A�� ��п�۴���п��
B�� ���ø����2mol/L������Һ
C�� ����18mol/L������Һ
D�� ���������Һ�м���������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������ȴ���Ӧ��ֻ���������ַе㲻ͬ��һ�ȴ�����������ǣ���
A�� ��CH3��2CHCH2CH2CH3 B�� ��CH3CH2��2CHCH3
C�� ��CH3��2CHCH��CH3��2 D�� ��CH3��3CCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Щ��ѧ��Ӧ���۷�Ӧ�����Ķ��٣���ֻ����ͬһ����
�ӷ���ʽ����ʾ�����и�������Һ�еķ�Ӧ��������һ������� �� ��
A��Ba(OH)2��H2SO4 B��KMnO4��H2O2
C��HCl��NaHCO3 D��Ca(HCO3)2��Ca(OH)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±��ж����ӷ���ʽ�����۲��������� �� ��
| ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
| A | NaClO��Һ��ͨ��������SO2��ClO����H2O��SO2===Cl����SO | �����Խ����в���������H�� |
| B | �����Ը��������Һ�ζ����2MnO | ��ȷ |
| C | NH4Al(SO4)2��Һ�е��뼸��NaOH��Һ��NH | ����OH�����Ⱥ�Al3����Ӧ����Al(OH)3���� |
| D | �ö��Ե缫���MgCl2��Һ��2Mg2����2H2O | ��ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и�������һ������ָ����Һ�д���������� �� ��
A��ʹ��̪���ɫ����Һ�У�Na����Al3����SO42����Cl��
B��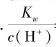 =1��10��13mol·L��1����Һ�У�NH4����Ca2����Cl����NO3��
=1��10��13mol·L��1����Һ�У�NH4����Ca2����Cl����NO3��
C����Al��Ӧ�ܷų�H2����Һ�У�Fe2����K����NO3����SO42��
D��ˮ�����c(H��)=1��10��13mol·L��1����Һ�У�K����Na����AlO2����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ�м����������ʣ���ʹˮ�ĵ���ƽ�ⷢ���ƶ����ǣ���
A�� NaCl B�� NaOH C�� �ƾ� D�� CH3COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧѧϰ���о���˵���������(����)
A����ѧģ�������ڽ���һЩ��ѧ����
B�������غ㶨���Ǵ���ʵ����ʵ���ܽ�
C����ѧ������ļ��趼�ܱ�ʵ��֤ʵ
D����ѧ����ԭ����Ӧ������һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ר��Ԥ�⣺��δ��30���ڣ�ȫ���֡�ʮ�����˼����������У��������������ü����������ڵڶ�λ���������ǷŴ��ط�����Դ�������������Դ��������������������ѭ�����õ�һ����Ҫԭ��
��1���������ǷŴ��ط�����Դ���������ѧ�Ƕȣ�̸̸�����仰�����⡣
_________________________________________________________________��
��2���������������ù����У������ǽ��������ࡣ���������ɷ�Ϊ������
���л����������鲣���������������������Ͼɷ�װ������������������
��3���л�������������֮һ�Ƿ��շ��硣���л�������ƽ����ѧ���Ϊ
CxHyOz�����л������ڷ��չ����з����Ļ�ѧ��Ӧ����ʽΪ��
_________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com