
ijŠ£»ÆѧŹµŃéŠĖȤŠ”×éĪŖĮĖĢ½¾æŌŚŹµŃéŹŅÖʱøCl2µÄ¹ż³ĢÖŠÓŠĖ®ÕōĘųŗĶHCl»Ó·¢³öĄ“£¬Ķ¬Ź±Ö¤Ć÷ĀČĘųµÄijŠ©ŠŌÖŹ£¬¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ(Ö§³ÅÓƵÄĢś¼ÜĢØŹ”ĀŌ)£¬Ķź³ÉĻĀĮŠĪŹĢā”£
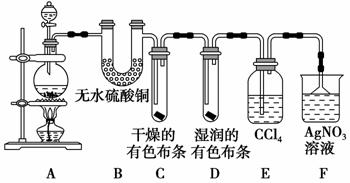
(1)×°ÖĆB”¢EµÄ×÷ÓĆ·Ö±šĪŖ_________________________________£¬
________________________________ӣ
(2)×°ÖĆC”¢DµÄ×÷ÓĆ·Ö±šĪŖ________________________________________£¬
________________________________ӣ
(3)×°ÖĆFÖŠAgNO3ČÜŅŗµÄ×÷ÓĆŹĒ________£¬µ¹ÖĆĀ©¶·µÄ×÷ÓĆŹĒ________”£
(4)ŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄŹµŃéӊȱĻŻ£¬²»ÄÜČ·±£×īÖÕĶØČėAgNO3ČÜŅŗÖŠµÄĘųĢåÖ»ÓŠŅ»ÖÖ”£ĪŖĮĖČ·±£ŹµŃé½įĀŪµÄæÉææŠŌ£¬Ö¤Ć÷×īÖÕĶØČėAgNO3ČÜŅŗµÄĘųĢåÖ»ÓŠŅ»ÖÖ£¬ŅŅĶ¬Ń§Ģį³öŌŚÄ³Į½øö×°ÖĆÖ®¼ä¼ÓŅ»øö×°ÖĆ”£ÄćČĻĪŖøĆ×°ÖĆ¼ÓŌŚ________Óė________Ö®¼ä(Ģī×°ÖĆø÷²æ·Ö×ÖÄø)£¬×°ÖĆÖŠÓ¦·ÅČė________£¬×÷ÓĆŹĒ________________”£
 Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µāŹĒČĖĢå±ŲŠėµÄŌŖĖŲÖ®Ņ»£¬ŗ£ŃóÖ²ĪļČēŗ£“ų”¢ŗ£ŌåÖŠŗ¬ÓŠ·įø»µÄ”¢ŅŌµāĄė×ÓŠĪŹ½“ęŌŚµÄµāŌŖĖŲ”£ŌŚŹµŃéŹŅÖŠ£¬“Óŗ£ŌåĄļĢįČ”µāµÄĮ÷³ĢŗĶŹµŃé×°ÖĆČēĻĀ£ŗ
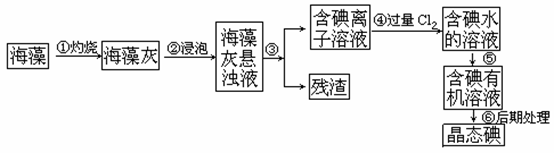
£Ø1£©Öø³öÉĻŹöĢįČ”µāµÄ¹ż³ĢÖŠÓŠ¹ŲŹµŃé²Ł×÷µÄĆū³Ę£ŗ²½Öč¢Ū________£¬²½Öč¢Ż_______”£
£Ø2£©Š“³ö²½Öč¢Ü¶ŌÓ¦·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ____________________ __”£
£Ø3£©ĢįČ”µāµÄ¹ż³ĢÖŠ£¬æɹ©Ń”ŌńµÄÓŠ»śŹŌ¼ĮŹĒ_______________”£
A. ¾Ę¾« B. “×Ėį C. ĖÄĀČ»ÆĢ¼ D. ±½
£Ø4£©ĪŖĮĖŹ¹ŗ£Ōå»ŅÖŠµÄµāĄė×Ó×Ŗ»ÆĪŖµāµÄÓŠ»śČÜŅŗ£¬¼“Ķź³É²½Öč¢ŪÖĮ¢Ż,ŹµŃéŹŅĄļÓŠÉÕ±”¢²£Į§°ō”¢¼ÆĘųĘ攢¾Ę¾«µĘ”¢µ¼¹Ü”¢Ō²µ×ÉÕĘ攢ŹÆĆŽĶų”¢ŅŌ¼°±ŲŅŖµÄ¼Š³ÖŅĒĘ÷ŗĶĪļĘ·£¬ÉŠČ±ÉŁµÄ²£Į§ŅĒĘ÷ŹĒ____________________________________”£
£Ø5£©“Óŗ¬µāµÄÓŠ»śČܼĮÖŠĢįČ”µāŗĶ»ŲŹÕÓŠ»śČܼĮ£¬»¹ŠčŅŖ¾¹żÕōĮó”£Öø³öĻĀĶ¼ŹµŃé×°ÖĆÖŠ“ęŌŚµÄ“ķĪóÖ®“¦£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ±ä»Æ¹ż³ĢŠčŅŖĪüŹÕÄÜĮæµÄŹĒ
A£®ĒāĘųĒņ·¢Éś±¬ÕØ B£®ĻņĪŪČ¾µÄŗÓĖ®ÖŠĶ¶·ÅÉśŹÆ»Ņ
C.  D.
D. 
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æÕĘų“µ³ö·Ø¹¤ŅÕ£¬ŹĒÄæĒ°”°ŗ£Ė®Ģįäå”±µÄ×īÖ÷ŅŖ·½·ØÖ®Ņ»”£Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ
ŗ£Ė®




 ””””
””””
(1)²½Öč¢ŪµÄĄė×Ó·½³ĢŹ½£ŗ_________________________________”£
(2)²½Öč¢ŻÖŠäåÕōĘųĄäÄżŗóµĆµ½ŅŗäåÓėäåĖ®µÄ»ģŗĻĪļ£¬æÉĄūÓĆĖüĆĒµÄĻą¶ŌĆܶČĻą²īŗÜ“óµÄĢŲµć½ųŠŠ·ÖĄė”£·ÖĄėŅĒĘ÷µÄĆū³ĘŹĒ________”£
(3)äåĖ®»ģŗĻĪļ¢ņÖŠČÜÓŠŅ»¶ØĮæµÄĀČĘų£¬Ņņ“ĖŌŚ²½Öč¢ŻÖŠæɽ«ÕōĮó²śÉśµÄĘųĢåĶعż________(ĢīŹŌ¼ĮĆū³Ę)ČÜŅŗ£¬ŅŌ³żČ„ĀČĘų”£
(4)²½Öč¢ŻµÄÕōĮó¹ż³ĢÖŠ£¬ĪĀ¶ČÓ¦æŲÖĘŌŚ80”«90 ”ę”£ĪĀ¶Č¹żøß»ņ¹żµĶ¶¼²»ĄūÓŚÉś²ś£¬Ēė½āŹĶŌŅņ£ŗ________________________________________________”£
(5)²½Öč¢ŁÖŠÓĆĮņĖįĖį»ÆæÉĢįøßCl2µÄĄūÓĆĀŹ£¬ĄķÓÉŹĒ___________ __________________________________________________________”£
(6)ĢįČ”äåµ„ÖŹ£¬²ÉÓĆÕōĮó”°äåĖ®»ģŗĻĪļ¢ņ”±¶ų²»ŹĒÕōĮó”°äåĖ®»ģŗĻĪļ¢ń”±£¬ĒėĖµĆ÷ŌŅņ£ŗ_________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŠĖȤŠ”×éĄūÓĆMnO2ŗĶÅØHCl¼°ČēĶ¼×°ÖĆÖʱøCl2”£ĻĀĮŠ·ÖĪöÖŠ²»ÕżČ·µÄŹĒ (””””)”£
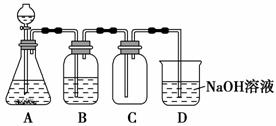
A£®AÖŠÓ¦ÓĆ·ÖŅŗĀ©¶·“śĢę³¤¾±Ā©¶·
B£®A֊ȱɣ¼ÓČČ×°ÖĆ
C£®BÖŠŹ¢·ÅµÄNaOHČÜŅŗæÉŅŌ¾»»ÆCl2
D£®DÖŠµÄµ¼¹ÜæŚČōĮ¬½Óµ¹ÖĆĀ©¶·æÉ·ĄÖ¹µ¹Īü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬ĻĀĮŠ²»·¢Éś·“Ó¦µÄŅ»×éĪļÖŹŹĒ (””””)”£
¢Ł¹čÓėNaOHČÜŅŗ””¢Ś¹čÓėŃĪĖį””¢Ū¹čÓėĒā·śĖį””¢Ü¶žŃõ»Æ¹čÓėĢ¼ĖįÄĘ””¢Ż¶žŃõ»Æ¹čÓėNaOHČÜŅŗ””¢Ž¶žŃõ»Æ¹čÓėÅØĻõĖį
A£®¢Ł¢Ś¢Ü”” B£®¢Ū¢Ü¢Ž
C£®¢Ś¢Ż¢Ž”” D£®¢Ś¢Ü¢Ž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹čŹĒĪŽ»ś·Ē½šŹō²ÄĮĻµÄÖ÷½Ē£¬¹čµÄŃõ»ÆĪļŗĶ¹čĖįŃĪŌ¼Õ¼µŲæĒÖŹĮæµÄ90%ŅŌÉĻ”£
(1)ĻĀĮŠĪļÖŹ²»ŹōÓŚ¹čĖįŃĪµÄŹĒ________”£
A£®ĢÕ“É”” B£®²£Į§
C£®Ė®Äą”” D£®ÉśŹÆ»Ņ
(2)SiO2ŹĒ²£Į§µÄÖ÷ŅŖ³É·ÖÖ®Ņ»£¬SiO2ÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________£¬¹¤ŅÕŹ¦³£ÓĆ________(ĢīĪļÖŹĆū³Ę)Ą“µńæĢ²£Į§”£
(3)ÓĆNa2SiO3Ė®ČÜŅŗ½žÅŻ¹żµÄĆŽ»Ø²»Ņ×Č¼ÉÕ£¬ĖµĆ÷Na2SiO3æÉÓĆ×÷________”£Na2SiO3æÉĶعżSiO2Óė“æ¼ī»ģŗĻøßĪĀČŪČŚ·“Ó¦ÖĘµĆ£¬øßĪĀČŪČŚ“æ¼īŹ±ĻĀĮŠŪįŪöæÉŃ”ÓƵďĒ________”£
A£®ĘÕĶز£Į§ŪįŪö”” B£®ŹÆÓ¢²£Į§ŪįŪö
C£®Ńõ»ÆĀĮŪįŪö”” D£®ĢśŪįŪö
(4)¹¤ŅµÉĻ³£ĄūÓĆ·“Ó¦2C£«SiO2 Si£«2CO”üÖʱø¹čµ„ÖŹ£¬øĆ·“Ó¦ÖŠĖłŗ¬ŌŖĖŲ»ÆŗĻ¼ŪÉżøßµÄĪļÖŹŹĒ________(Ģī»ÆѧŹ½£¬ĻĀĶ¬)£¬Ńõ»Æ¼ĮŹĒ________”£
Si£«2CO”üÖʱø¹čµ„ÖŹ£¬øĆ·“Ó¦ÖŠĖłŗ¬ŌŖĖŲ»ÆŗĻ¼ŪÉżøßµÄĪļÖŹŹĒ________(Ģī»ÆѧŹ½£¬ĻĀĶ¬)£¬Ńõ»Æ¼ĮŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½šŹō²ÄĮĻŌŚČÕ³£Éś»īŅŌ¼°Éś²śÖŠÓŠ×Źć·ŗµÄÓ¦ÓĆ”£ĻĀĮŠ¹ŲÓŚ½šŹōµÄŅ»Š©Ėµ·Ø²»ÕżČ·µÄŹĒ (””””)”£
A£®ŗĻ½šµÄŠŌÖŹÓėĘä³É·Ö½šŹōµÄŠŌÖŹ²»ĶźČ«ĻąĶ¬
B£®¹¤ŅµÉĻ½šŹōMg”¢Cu¶¼ŹĒÓĆČČ»¹Ō·ØÖʵƵÄ
C£®½šŹōŅ±Į¶µÄ±¾ÖŹŹĒ½šŹōŃōĄė×ӵƵ½µē×Ó±ä³É½šŹōŌ×Ó
D£®Ō½»īĘĆµÄ½šŹōŌ½ÄŃŅ±Į¶
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«Ņ»¶ØĮæµÄNa2O2ŗĶNaHCO3»ģŗĻŗó£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČ³ä·Ö·“Ó¦£¬ÅųöĘųĢ壬ĄäČ“ŗóÓŠ¹ĢĢåĪļÖŹŹ£Óą£¬ĻĀĮŠŃ”Ļī²»ÕżČ·µÄŹĒ (””””)”£
| Na2O2/mol | NaHCO3/mol | Ź£ÓąµÄ¹ĢĢåĪļÖŹ | |
| A | 1 | 2 | Na2CO3 |
| B | 1.5 | 2 | Na2O2””Na2CO3 |
| C | 2 | 1 | Na2O2””NaOH””Na2CO3 |
| D | 2 | 2 | NaOH””Na2CO3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com