
����Ŀ��25 ��ʱ�������ΪVa��pH��a��ijһԪ��HA��Һ�����ΪVb��pH��b��ijһԪ��BOH��Һ��ϣ���ش�
��1����a + b��14��2Va��Vb����Ӧ��������ҺpH��7�������ɵ�����Һ�У�һ������ˮ������ӷ� ��ʽΪ_______
��2����a + b��12���������ᣬ����KOH����Ӧ��������ҺpH��7����Va��Vb�Ĺ�ϵ��_______
��3�����������ᣬ���ǰ�ˮ����Ӧ��������Һ������Ũ�ȴ�С��ϵ��������_________������ţ�
A��c(Cl��)��c(NH)��c(H+)��c(OH��) B��c(H+)��c(OH��)��c(Cl��)��c(NH)
C��c(NH)��c(Cl��)��c(OH��)��c(H+) D��c(Cl��)��c(H+)��c(NH)��c(OH��)
E��c(Cl��)��c(NH)��c(H+)��c(OH��)
��4�������Ǵ��ᣬ����NaOH���ҷ�Ӧ������Һ��c(CH3COO��)��c(H+)��������Һ���ܳ�_____������ţ�
A������ B������ C������ D�������
��5��25 ��ʱ�������Va��200 mL��pH��2��H2SO4��Һ�����Vb��10 mL��pH��11�İ�ˮ��Һ��ϣ�ǡ����ȫ��Ӧ����������£���ˮ�ĵ���ƽ�ⳣ����____________
���𰸡�A��+ H2O![]() HA + OH�� Vb��100 Va B D 5��10��6
HA + OH�� Vb��100 Va B D 5��10��6
��������
������ɷ�����Һ�����ʵijɷ֣��ٸ�����Һ����ԡ�����غ��������غ�ȷ�����Һ������Ũ�ȴ�С��
(1)pH=a�������Һ��c��H+��=10-pHmolL-1=10-amolL-1��a+b=14����pH=b�ļ���c��OH-��=10pH-14molL-1=10b-14molL-1=10-amolL-1���ɼ�����c��H+�������c��OH-����ȣ�2Va=Vb��Ϻ���Һ��pH=7��˵����ĵ���̶����ڼ�ĵ���̶ȣ���������һ��������һ������ˮ������ӷ���ʽΪ��A��+ H2O![]() HA + OH����
HA + OH����
(2)����c��H+��=10-pHmolL-1=10-amolL-1������c��OH-��=10pH-14molL-1=10b-14molL-1=10-��a+2��molL-1��pH=7ʱ����Ϊǿ��ǿ�Ӧ������Va 10-amolL-1=Vb10-��a+2��molL-1���ʴ�Ϊ�� Vb��100 Va��
(3)���������ᣬ���ǰ�ˮ�����������������ҺΪ�Ȼ�狀����ᣬ��Һ�����ԣ���Һ������Ũ�ȴ�СΪ�� c��Cl-��> c��NH4+��>c��H+��>c��OH-����c��Cl-��> c��H+��>c��NH4+��>c��OH-����A��C��ȷ��������Ͱ�ˮ��Ӧ����Һ�ʼ��������ʱ��ˮ��������Һ�д��ڵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-������OH-��>c��H+�����õ�c��NH4+��>c��Cl-������Һ������Ũ�ȴ�СΪ��c��NH4+��>c��Cl-��>c��OH-��>c��H+������C��ȷ������ҺpH��7����Ӧ����ҺΪ�Ȼ�狀Ͱ�ˮ�Ļ��Һ����Һ�д��ڵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-����c��OH-��=c��H+�����õ�c��NH4+��=c��Cl-������Һ������Ũ�ȴ�СΪ��c��NH4+��=c��Cl-��>c��OH-��=c��H+������E��ȷ����Һ����Ϊ�����˰�ˮ���ʲ����ܳ���c(H+)��c(Cl��)����B���ʴ�ΪB��
(4)��Ӧ����Һ��Ϊ������������������������������������ԣ�ǡ����ȫ��Ӧ���Ǽ�����ʼ��ԣ��κ�ʱ��c(CH3COO��)��c(H+)���ʴ�ѡD��
(5)��Ϊǡ����ȫ��Ӧ������n(NH3��H2O)=2n(H2SO4)=2![]() (0.2L
(0.2L![]() 10-2 molL-1/2)=0.002 mol��c(NH3��H2O)=0.001mol/0.01L=0.2 molL-1 ��pH��11�İ�ˮ��Һ�У�pOH=14-11=3�� c(OH-)=10-3 molL-1��NH3��H2O�ĵ���ƽ�ⳣ��ΪKb��
10-2 molL-1/2)=0.002 mol��c(NH3��H2O)=0.001mol/0.01L=0.2 molL-1 ��pH��11�İ�ˮ��Һ�У�pOH=14-11=3�� c(OH-)=10-3 molL-1��NH3��H2O�ĵ���ƽ�ⳣ��ΪKb��![]() =��10-3 molL-1��2/0.2 molL-1=5��10��6���ʴ�Ϊ��5��10��6��
=��10-3 molL-1��2/0.2 molL-1=5��10��6���ʴ�Ϊ��5��10��6��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������۵���ߵĽ���������Ҫ��ս�����ʡ���Ȼ��������Ҫ�����ں��ٿ��У�����Ҫ�ɷ��������̵������Σ�FeWO4��MnWO4������������Si��As�Ļ�����ɺ��ٿ�ұ���ٵĹ����������£�

��֪��
������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У��ٵĻ��ϼ�ֻ�������һ�������ı䡣
������������������ˮ��
��1����д��FeWO4�����������·�����ֽⷴӦ����Fe2O3�Ļ�ѧ����ʽ��______________________________________��
��2�����������������������Һ�м������к���pH=10����Һ�е�����������ΪSiO32�D��HAsO32�D��HAsO42�D�ȣ����������������У�����H2O2ʱ������Ӧ�����ӷ���ʽΪ_________������������Ҫ�ɷ���______________��
��3����֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С����ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ��������

��T1_____T2������>������<����T1ʱKsp��CaWO4��=______________��
������������Һ����ʯ����õ���������ƣ�������Ӧ�����ӷ���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����ij2 L�ܱ������м���һ�����Ĺ���A������B��������Ӧ
A(s)��2B(g) ![]() D(g)��E(g)����H��Q kJ��mol��1����T1 ��ʱ����Ӧ���е���ͬʱ���ø����ʵ����ʵ��������
D(g)��E(g)����H��Q kJ��mol��1����T1 ��ʱ����Ӧ���е���ͬʱ���ø����ʵ����ʵ��������
��ʱ��(min) ���ʵ���(mol)���������� | 0 | 10 | 20 | 30 | 40 | 50 |
B | 2.00 | 1.36 | 1.00 | 1.00 | 1.20 | 1.20 |
D | 0 | 0.32 | 0.50 | 0.50 | 0.60 | 0.60 |
E | 0 | 0.32 | 0.50 | 0.50 | 0.60 | 0.60 |
��1��T 1 ��ʱ���÷�Ӧ��ƽ�ⳣ��K��________��
��2��30 min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬���ݱ��е������жϸı������������________(����ĸ���)��
a��ͨ��һ������B
b������һ�����Ĺ���A
c���ʵ���С���������
d�����߷�Ӧ��ϵ�¶�
e��ͬʱ����0.2 mol B��0.1 mol D��0.1 mol E
��3�����ڸ÷�Ӧ���ø����ʱ�ʾ�ķ�Ӧ������ʱ��Ĺ�ϵʾ������Ϊͼ1�е�________(�����)��
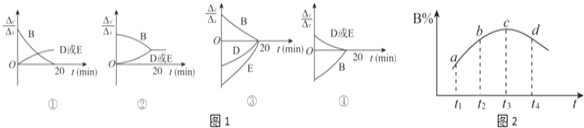
��4��ά��������������¶�T1 �����䣬����������м���1.60 mol B��0.20 mol D��0.20 mol E��n mol A���ﵽƽ����������20����ʱ�����ʵ�Ũ����ȫ��ͬ����Ͷ�����A�����ʵ���n��ȡֵ��Χ��________��
��5��ά��������������¶�T1 �����䣬�����ʵ���ʼ���ʵ���Ϊ��n(A)��1.0 mol��n(B)��3.0 mol��n(D)��a mol��n(E)��0 mol���ﵽƽ���n(E)��0.50 mol����a��________��
��6�������ܱ��������ȣ�ʵ����B��ת����B% ���¶ȱ仯��ʾ��ͼ��ͼ2��ʾ����ͼ��֪��Q_______0(��������������С����)��c��v��_______v��(��������������С��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaOH��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ��������1.16g��ɫ������������������Һ����μ���1.00mol/L HCl��Һ������HCl��Һ����������������Ĺ�ϵ��ͼ��ʾ���Իش�
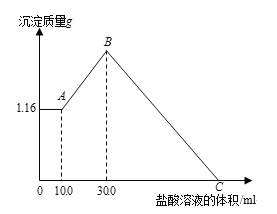
��1��д��A�㵽B�㷢����Ӧ�����ӷ���ʽ
��2��C�����������Һ�����Ϊ ��
��3��ԭ�������MgCl2�����ʵ����� ��NaOH�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵����ȷ����(����)
A. ���ں�������ϸ���һЩͭ�飬����Լ���������ǵĸ�ʴ
B. 2NO(g)��2CO(g)===N2(g)��2CO2(g)�ڳ��������Է����У���÷�Ӧ����H>0
C. ����0.1 mol��L��1Na2CO3��Һ��CO![]() ��ˮ��̶Ⱥ���Һ��pH������
��ˮ��̶Ⱥ���Һ��pH������
D. �����������Ҵ���������Ӧ(��H<0)����������Ũ���Ტ���ȣ��÷�Ӧ�ķ�Ӧ���ʺ�ƽ�ⳣ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���绯ѧ���������������ڼ�����NH3�ĺ���,�乤��ԭ����ͼ��ʾ,����NH3������Ϊ�����������ʡ�����˵��������ǣ� ��

A. ��Һ��OH-��缫a�ƶ�
B. �缫b�Ϸ�����ԭ��Ӧ
C. �����ĵ缫��ӦΪ2NH3-6e-+6OH-![]() N2+6H2O
N2+6H2O
D. ���۷�Ӧ���ĵ�NH3��O2�����ʵ���֮��Ϊ3��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������(��NO2��NO4��N2O5��)Ӧ�úܹ㣬��һ�������¿����ת����
(1) ��֪��2NO(g)��O2(g)===2NO2(g)����H1
NO(g)��O3(g)===NO2(g)��O2(g)����H2
2NO2(g)![]() N2O4(g)����H3
N2O4(g)����H3
2N2O5(g)===4NO2(g)��O2(g)����H4
��ӦN2O4(g)��O3(g)===N2O5(g)��O2(g)�Ħ�H��__________��
(2)��N2O5��һ�������·����ֽ⣺2N2O5(g)===4NO2(g)��O2(g)��ij�¶��²�ú����ܱ�������N2O5Ũ����ʱ��ı仯���±���
t/min | 0.00 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 |
c(N2O5)/ (mol��L��1) | 1.00 | 0.71 | 0.50 | 0.35 | 0.25 | 0.17 |
�跴Ӧ��ʼʱ��ϵѹǿΪp0����2.00 minʱ��ϵѹǿΪp����p��p0��________��1.00��3.00 min�ڣ�O2��ƽ����Ӧ����Ϊ________��
(3)N2O4��NO2֮����ڷ�ӦN2O4(g)![]() 2NO2(g)����һ������N2O4��������ܱ������У������ƽ��ת����[��(N2O4)]���¶ȱ仯��ͼ��ʾ��
2NO2(g)����һ������N2O4��������ܱ������У������ƽ��ת����[��(N2O4)]���¶ȱ仯��ͼ��ʾ��

��ͼ��a���Ӧ�¶��£���֪N2O4����ʼѹǿp0Ϊ108 kPa����ʽ������¶��·�Ӧ��ƽ�ⳣ��Kp��________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
����ͼ�Ʋ�N2O4(g)![]() 2NO2(g)�����ȷ�Ӧ���Ƿ��ȷ�Ӧ��˵������_______________����Ҫ���N2O4ת���ʣ����ı䷴Ӧ�¶��⣬������ʩ��____________(Ҫ��д������)��
2NO2(g)�����ȷ�Ӧ���Ƿ��ȷ�Ӧ��˵������_______________����Ҫ���N2O4ת���ʣ����ı䷴Ӧ�¶��⣬������ʩ��____________(Ҫ��д������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ִ���ҵ�ķ�չ����Դ�����Ѿ�Խ��Խ�������ǵ����ӡ���ѧ��Ԥ�ԣ�δ���������ȼ������ɫֲ�����ֲ��Ľո�(��Ҫ�ɷ�����ά��)���ʵ��Ĵ���ˮ��������ǣ��ٽ�������ת��Ϊ�Ҵ�������ȼ�ϡ�
��1��д����ɫֲ��Ľո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
��____________________________________________��
��____________________________________________��
��2���Ҵ�������ȼ���⣬�����������ϳ������л����ͼ��Ҫ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ������ͼ��������Ӧ���ʵĽṹ��ʽ___________________________��
![]()
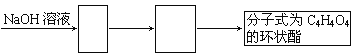
��3��д������ת����ϵͼ����CH2OHCH2OH![]() C4H4O4�Ļ�ѧ����ʽ ���л����ýṹ��ʽ��ʾ��________________________________________________________________��
C4H4O4�Ļ�ѧ����ʽ ���л����ýṹ��ʽ��ʾ��________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com