
”¾ĢāÄæ”æĪļÖŹµÄĮæŹĒøßÖŠ»Æѧ³£ÓƵÄĪļĄķĮ棬ĒėĶź³ÉŅŌĻĀÓŠ¹Ų¼ĘĖć”£
£Ø1£©ŗ¬0.4 mol Al3£«µÄAl2(SO4)3ÖŠĖłŗ¬µÄSO42£µÄĪļÖŹµÄĮæŹĒ___________”£
£Ø2£©___________molH2O2Ėłŗ¬Ō×ÓŹżÓė0.2molH3PO4Ėłŗ¬Ō×ÓŹżĻąµČ”£
£Ø3£©Ä³ĮņĖįÄĘČÜŅŗÖŠŗ¬ÓŠ3.01”Į1022øöNa+£¬ŌņČÜŅŗÖŠSO42£µÄĪļÖŹµÄĮæŹĒ___________”£
£Ø4£©0.7 mol H2OµÄÖŹĮæĪŖ___________”£
£Ø5£©483gNa2SO4”¤10H2OÖŠĖłŗ¬µÄNa+µÄĪļÖŹµÄĮæŹĒ___mol£¬Ėłŗ¬H2O·Ö×ӵďżÄæŹĒ___øö”£
£Ø6£©ÖŹĮæĻąĶ¬µÄH2”¢NH3”¢SO2”¢O3ĖÄÖÖĘųĢåÖŠ£¬ŗ¬ÓŠ·Ö×ÓŹżÄæ×īÉŁµÄŹĒ____________”£
£Ø7£©aøöXŌ×ÓµÄ×ÜÖŹĮæĪŖbg£¬ŌņXµÄĻą¶ŌŌ×ÓÖŹĮææÉŅŌ±ķŹ¾ĪŖ___________
£Ø8£©ĻÖÓŠmgijĘųĢ壬ĖüŹĒČżŌ×Ó·Ö×Ó£¬ĘäĦ¶ūÖŹĮæĪŖMg”¤mol-1”£Čō°¢·ü¼ÓµĀĀŽ³£ŹżÓĆNA±ķŹ¾£¬ĒėÓĆŅŌÉĻ·ūŗż°ĻąÓ¦Źż×ÖĢīŠ“ĻĀĮŠæÕøń”£
¢ŁøĆĘųĢåµÄĪļÖŹµÄĮæĪŖ___________mol”£
¢ŚøĆĘųĢåĖłŗ¬Ō×Ó×ÜŹżĪŖ___________øö”£
”¾“š°ø”æ0.6 mol0.40.025mol12.6g315NASO2bNA/am/M3mNA/M
”¾½āĪö”æ
£Ø1£©Al2£ØSO4£©3ÖŠn£ØAl3+£©£ŗn£ØSO42-£©=2£ŗ3£¬¹Źŗ¬0.4molAl3+µÄAl2£ØSO4£©3ÖŠĖłŗ¬SO42-µÄĪļÖŹµÄĮæŹĒ0.4mol”Į![]() =0.6mol£¬¹Ź“š°øĪŖ£ŗ0.6mol£¬
=0.6mol£¬¹Ź“š°øĪŖ£ŗ0.6mol£¬
£Ø2£©1øöĮ×Ėį·Ö×Óŗ¬ÓŠ8øöĒāŌ×Ó£¬Ōņ0.2molH3PO4ŗ¬ÓŠ1øö¹żŃõ»ÆĒā·Ö×Óŗ¬ÓŠ4øöĒāŌ×Ó£¬Ōņŗ¬ÓŠ1.6molŌ×ÓµÄH2O2µÄĪļÖŹµÄĮæĪŖ£ŗ![]() =0.4mol£¬¹Ź“š°øĪŖ£ŗ0.4£»
=0.4mol£¬¹Ź“š°øĪŖ£ŗ0.4£»
£Ø3£©ĆæøöĮņĖįÄĘ»ÆѧŹ½ÖŠĮņĖįøłĄė×ÓŗĶÄĘĄė×ÓµÄøöŹżÖ®±ČĪŖ1£ŗ2£¬ŗ¬ÓŠ3.01”Į1023øöNa+£¬ŌņøĆČÜŅŗÖŠSO42-µÄøöŹżĪŖ![]() ”Į3.01”Į1023£¬ŌņĮņĖįøłĄė×ÓµÄĪļÖŹµÄĮæn=
”Į3.01”Į1023£¬ŌņĮņĖįøłĄė×ÓµÄĪļÖŹµÄĮæn=![]() =0.25mol£¬¹Ź“š°øĪŖ£ŗ0.25mol£»
=0.25mol£¬¹Ź“š°øĪŖ£ŗ0.25mol£»
£Ø4£©0.7molĖ®µÄÖŹĮæĪŖ£ŗ18g/mol”Į0.7mol=12.6g£¬¹Ź“š°øĪŖ£ŗ12.6g£»
£Ø5£©483gNa2SO410H2OµÄĪļÖŹµÄĮæ=![]() =1.5mol£¬Na+µÄĪļÖŹµÄĮæĪŖNa2SO410H2OµÄ2±¶ĪŖ1.5mol”Į2=3mol£¬H2O·Ö×ÓµÄĪļÖŹµÄĮæĪŖNa2SO410H2OµÄ10±¶ĪŖ1.5mol”Į10=15mol£¬¹ŹĖłŗ¬Ė®·Ö×ÓŹżÄæĪŖ15NA£¬¹Ź“š°øĪŖ£ŗ3£»15NA£»
=1.5mol£¬Na+µÄĪļÖŹµÄĮæĪŖNa2SO410H2OµÄ2±¶ĪŖ1.5mol”Į2=3mol£¬H2O·Ö×ÓµÄĪļÖŹµÄĮæĪŖNa2SO410H2OµÄ10±¶ĪŖ1.5mol”Į10=15mol£¬¹ŹĖłŗ¬Ė®·Ö×ÓŹżÄæĪŖ15NA£¬¹Ź“š°øĪŖ£ŗ3£»15NA£»
£Ø6£©øł¾Żn=![]() ”¢N=nNAæÉÖŖ£¬ÖŹĮæĻąµČ£¬Ä¦¶ūÖŹĮæŌ½“ó£¬ĘųĢåµÄĪļÖŹµÄĮæŌ½Š”£¬ŗ¬ÓŠµÄ·Ö×ÓŹżŌ½ÉŁ£¬ĖÄÖÖĘųĢåÖŠ£¬Ä¦¶ūÖŹĮæ×ī“óµÄŹĒ¶žŃõ»ÆĮņ£¬ĖłŅŌŗ¬ÓŠ·Ö×ÓŹż×īÉŁµÄŹĒ¶žŃõ»ÆĮņ£¬¹Ź“š°øĪŖ£ŗSO2£»
”¢N=nNAæÉÖŖ£¬ÖŹĮæĻąµČ£¬Ä¦¶ūÖŹĮæŌ½“ó£¬ĘųĢåµÄĪļÖŹµÄĮæŌ½Š”£¬ŗ¬ÓŠµÄ·Ö×ÓŹżŌ½ÉŁ£¬ĖÄÖÖĘųĢåÖŠ£¬Ä¦¶ūÖŹĮæ×ī“óµÄŹĒ¶žŃõ»ÆĮņ£¬ĖłŅŌŗ¬ÓŠ·Ö×ÓŹż×īÉŁµÄŹĒ¶žŃõ»ÆĮņ£¬¹Ź“š°øĪŖ£ŗSO2£»
£Ø7£©aøöXŌ×ÓµÄ×ÜÖŹĮæĪŖbg£¬ŌņNAøöŌ×ÓµÄÖŹĮæĪŖNA”Į![]() g£¬ŹżÖµÉĻµČÓŚĘäĻą¶ŌŌ×ÓÖŹĮ棬¹ŹøĆŌ×ÓĻą¶ŌŌ×ÓÖŹĮæĪŖ
g£¬ŹżÖµÉĻµČÓŚĘäĻą¶ŌŌ×ÓÖŹĮ棬¹ŹøĆŌ×ÓĻą¶ŌŌ×ÓÖŹĮæĪŖ![]() £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ![]() £»
£»
(8)¢Łm gijĘųĢåµÄĪļÖŹµÄĮæĪŖ![]() =
=![]() mol£¬¹Ź“š°øĪŖ£ŗ
mol£¬¹Ź“š°øĪŖ£ŗ![]() £»
£»
¢ŚŅņĪŖŅ»øö·Ö×ÓÖŠŗ¬ČżøöŌ×Ó£¬ĖłŅŌŗ¬ÓŠµÄŌ×ÓŹżĪŖ·Ö×ÓŹżµÄ2±¶£¬¼“ĪŖ3”Į![]() mol”ĮNAmol-1=
mol”ĮNAmol-1=![]() NA£¬¹Ź“š°øĪŖ£ŗ
NA£¬¹Ź“š°øĪŖ£ŗ![]() NA”£
NAӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠmgijX2ĘųĢ壬ĖüµÄĦ¶ūÖŹĮæĪŖM g/mol£¬°¢·ü¼ÓµĀĀŽ³£ŹżÓĆNA±ķŹ¾£¬Ōņ£ŗ
£Ø1£©øĆĘųĢåµÄĪļÖŹµÄĮæĪŖ________mol”£
£Ø2£©Ņ»øöXŌ×ÓµÄÖŹĮæ_________g”£
£Ø3£©øĆĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________L”£
£Ø4£©øĆĘųĢåČÜÓŚĖ®ŗóŠĪ³ÉVLČÜŅŗ£¬ĘäČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________mol”¤L£1”£
£Ø5£©øĆĘųĢåČÜÓŚ1LĖ®ÖŠ(²»æ¼ĀĒ·“Ó¦)£¬ĖłµĆČÜŅŗµÄĆܶČĪŖ¦Ńg/cm3£¬ŌņøĆČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ__________mol”¤L£1”£
£Ø6£©ĻąĶ¬×“æöĻĀ£¬ČōX2ÓėµŖĘųŅŌĢå»ż±Č1:4»ģŗĻ£¬øĆ»ģŗĻĘųĢåĻą¶ŌĒāĘųĆܶČĪŖ14.4£¬X2µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ_______”£
£Ø7£©Čō±źæöĻĀX2µÄĆܶČĪŖ1.25 g/L£¬XŌ×Ó×īĶā²ćÓŠ_____øöµē×Ó”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČéĖįµÄ¹¤ŅµÉś²śæÉŅŌ²ÉÓĆÉśĪļ·¢½Ķ·Ø£¬Ņ²æÉŅŌ²ÉÓĆÓŠ»śŗĻ³É·Ø£¬ĘäÖŠµÄŅ»ÖÖ·½·ØŹĒ±ūĖį·Ø”£
¢ń.ÉśĪļ·¢½Ķ·Ø
![]()
£Ø1£©¼ģŃéµķ·ŪŹĒ·ńĶźČ«Ė®½ā£¬æÉŅŌŃ”ÓƵÄŅ»ÖÖŹŌ¼ĮŹĒ__________£ØĢīŠņŗÅ£©”£
a.äåĖ® b.Ņų°±ČÜŅŗ c.µā¾Ę d.µā»Æ¼Ųµķ·ŪČÜŅŗ
£Ø2£©ŅŃÖŖĘĻĢŃĢĒŌŚČéĖį¾ś×÷ÓĆĻĀæÉ×Ŗ»ÆĪŖČéĖį(C3H6O3)”£
¢ŁŠ“³öĘĻĢŃĢĒÓėŅų°±ČÜŅŗ·“Ó¦£¬ŌŁĖį»ÆµÄÓŠ»ś²śĪļµÄ½į¹¹¼ņŹ½£ŗ___________________”£
¢ŚČéĖįŌŚCu×÷“߻ƼĮŹ±æɱ»Ńõ»Æ³É±ūĶŖĖį(![]() )£¬ÓÉŅŌÉĻŹĀŹµĶĘÖŖČéĖįµÄ½į¹¹¼ņŹ½ĪŖ_______”£
)£¬ÓÉŅŌÉĻŹĀŹµĶĘÖŖČéĖįµÄ½į¹¹¼ņŹ½ĪŖ_______”£
¢ŪČéĖįŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļµÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________
¢ÜČéĖįŌŚÅØH2SO4¼ÓČČĢõ¼žĻĀæÉŅŌÉś³ÉŅ»ÖÖÄÜŹ¹äåĖ®ĶŹÉ«µÄÓŠ»śĪļA”£ AŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌŗĻ³ÉŅ»ÖÖ³£¼ūµÄÓŠ»śøß·Ö×Ó»ÆŗĻĪļB£¬BµÄ½į¹¹¼ņŹ½ĪŖ____________________________”£
¢ņ.±ūĖįŗĻ³É·Ø

£Ø3£©·“Ó¦IµÄ·“Ó¦ĄąŠĶŹĒ________£¬½ųŠŠ·“Ó¦¢ņŹ±£¬ŠčŅŖ¼ÓČė¹żĮæŅŅ“¼£¬ÕāŃł×öµÄÄæµÄŹĒ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅ»ÖÖĄūÓƵē½āĆĢŃō¼«Äą(Ö÷ŅŖ³É·ÖMnO2”¢MnO)ÖʱøMnO2µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
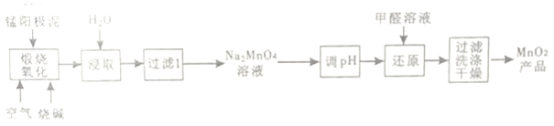
(1)”°ģŃÉÕŃõ»Æ”±Ź±£¬1mol MnOģŃÉÕĶźČ«×Ŗ»ÆĪŖNa2MnO4Ź§Č„µē×ÓµÄĪļÖŹµÄĮæĪŖ___________£»MnO2ģŃÉÕ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________”£
(2)”°½žČ””±Ź±£¬ĪŖĢįøßNa2MnO4µÄ½žČ”ĀŹ£¬æɲÉČ”µÄ“ėŹ©ÓŠ____________”¢____________(ĮŠ¾Ł2µć)
(3)”°µ÷pH”±ŹĒ½«ČÜŅŗpH µ÷ÖĮŌ¼ĪŖ10£¬·ĄÖ¹pH½ĻµĶŹ±Na2MnO4×ŌÉķ·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬Éś³ÉMnO2ŗĶ___________£»Š“³öÓĆpHŹŌÖ½²ā¶ØČÜŅŗpHµÄ²Ł×÷_______________”£
(4)”°»¹Ō”±Ź±ÓŠĪŽ»śŗ¬ŃõĖįŃĪÉś³É£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________”£
(5)²ā¶Ø²śĘ·ÖŠMnO2ÖŹĮæ·ÖŹżµÄ²½ÖčČēĻĀ£ŗ
²½Öč1. ×¼Č·³ĘČ”mg²śĘ·£¬¼ÓČėc1mol”¤L-1Na2C2O4ČÜŅŗV1mL (¹żĮæ)¼°ŹŹĮæµÄĻ”ĮņĖį£¬Ė®Ō”¼ÓČČÖó·ŠŅ»¶ĪŹ±¼ä”£(ŅŃÖŖ£ŗNa2C2O4+2H2SO4+MnO2=MnSO4+2CO2”ü+2H2O+Na2SO4)
²½Öč2. Č»ŗóÓĆc2mol”¤L-1KMnO4±ź×¼ČÜŅŗµĪ¶ØŹ£ÓąµÄNa2C2O4µĪ¶ØÖĮÖÕµćŹ±ĻūŗÄKMnO4±ź×¼ČÜŅŗV2mL”£(ŅŃÖŖ£ŗ5H2C2O4+2KMnO4+3H2SO4=2MnSO4+10CO2”ü+K2SO4+8H2O)
²½Öč2“ļµĪ¶ØÖÕµćŹ±ÅŠ¶ĻŅĄ¾ŻŹĒ_____________£»²śĘ·ÖŠMnO2µÄÖŹĮæ·ÖŹżĪŖ¦Ų(MnO2)=____________(ĮŠ³ö¼ĘĖćµÄ±ķ“ļŹ½¼“æÉ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. °¢·ü¼ÓµĀĀŽ³£ŹżŹĒ12gĢ¼ÖŠĖłŗ¬ÓŠµÄĢ¼Ō×ÓŹż
B. ŅŃÖŖĪ¢Į£øöŹż£¬æÉĶعż°¢·ü¼ÓµĀĀŽ³£Źż¼ĘĖć³öĪ¢Į£µÄĪļÖŹµÄĮæ
C. °¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµŹĒ6.02”Į1023
D. °¢·ü¼ÓµĀĀŽ³£ŹżµÄ·ūŗÅĪŖNA£¬Ķس£ÓĆ6.02”Į1023±ķŹ¾
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A. ±ź×¼×“æöĻĀ£¬2.24LŅŅĶéÓė2.24L±½ŗ¬C-H¼üŹż¾łĪŖ0.6NA
B. 16.8gFeÓė×ćĮæµÄĖ®ÕōĘų¼ÓČČ³ä·Ö·“Ó¦£¬×ŖŅʵē×ÓŹżĪŖ0.8NA
C. 25CŹ±£¬1LpH=7µÄCH3COONH4ČÜŅŗÖŠŗ¬NH4+ŹżŅ»¶ØĪŖ1.0”Į10-7NA
D. 0.2molCO2Óė0.1molCŌŚĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ӧɜ³ÉCO·Ö×ÓŹżĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijŹµŃ銔×éĪŖĮĖĢ½¾æSO2µÄŠŌÖŹ£¬Éč¼ĘĮĖČēĻĀ×°ÖĆ£¬

ŹµŃé²½Öč£ŗ
¢ŁĻČĮ¬½ÓŗĆ×°ÖĆ£¬¼ģ²éĘųĆÜŠŌ£¬ŌŁ¼ÓČėŹŌ¼Į£»
¢Ś¼ÓČČAŹŌ¹Ü£»
¢Ū½«ĶĖæĻņÉĻ³é¶ÆĄėæŖŅŗĆę”£
£Ø1£©AŹŌ¹ÜÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ______”£
£Ø2£©BŹŌ¹ÜÖŠµÄĻÖĻóŹĒ______”£
£Ø3£©ŹŌ¹ÜCĪŽĆ÷ĻŌĻÖĻó£¬Ä³Š”×éČ”Ņ»²æ·Ö·“Ó¦ŗóµÄČÜŅŗ£¬·Ö±šµĪ¼ÓŅŌĻĀŹŌ¼Į£¬ĒėÄćŌ¤²āÄÜ·ńÉś³É³Įµķ£¬ČōÉś³É³Įµķ£¬Š“³öÉś³É³ĮµķµÄ»ÆѧŹ½”£
¼ÓČėŹŌ¼Į | ÄÜ·ńÉś³É³Įµķ | ³ĮµķµÄ»ÆѧŹ½ |
ĀČĖ® | _____________ | __________ |
°±Ė® | __________ | ___________ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅŠčÓĆ![]() µÄĮņĖįĶČÜŅŗ£¬ŅŌĻĀ²Ł×÷ÕżČ·µÄŹĒ
µÄĮņĖįĶČÜŅŗ£¬ŅŌĻĀ²Ł×÷ÕżČ·µÄŹĒ![]() ””””
””””![]()
A. ½«![]() µØ·ÆÅä³É500mLČÜŅŗ
µØ·ÆÅä³É500mLČÜŅŗ
B. ½«![]() µØ·ÆČÜÓŚÉŁĮæĖ®ÖŠ£¬ŌŁÓĆĖ®Ļ”ŹĶÖĮ500mL
µØ·ÆČÜÓŚÉŁĮæĖ®ÖŠ£¬ŌŁÓĆĖ®Ļ”ŹĶÖĮ500mL
C. ³ĘČ”![]() ĮņĖįĶ£¬¼ÓČė500mLĖ®
ĮņĖįĶ£¬¼ÓČė500mLĖ®
D. ŌŚ![]()
![]() ČÜŅŗÖŠ¼ÓČė400mLĖ®
ČÜŅŗÖŠ¼ÓČė400mLĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijĶ¬Ń§Éč¼ĘČēĻĀŹµŃé·½°ø£¬ŅŌ·ÖĄėKClŗĶBaCl2Į½ÖÖ¹ĢĢå»ģŗĻĪļ£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

¹©Ń”ŹŌ¼Į£ŗNa2SO4ČÜŅŗ”¢K2CO3ČÜŅŗ”¢K2SO4ČÜŅŗ”¢ŃĪĖį
£Ø1£©²Ł×÷¢ŚµÄĆū³ĘŹĒ______________£¬ŹŌ¼ĮaµÄČÜÖŹŹĒ_______________£ØĢī»ÆѧŹ½£©
£Ø2£©¼ÓČėŹŌ¼ĮbĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________________________”£
£Ø3£©øĆ·½°øÄܲ»ÄÜ“ļµ½ŹµŃéÄæµÄ£ŗ_____________£¬Čō²»ÄÜ£¬Ó¦ČēŗĪøĽų£æ£ØČōÄÜ£¬“ĖĪŹ²»ÓĆ»Ų“š£©________________________________________”£
£Ø4£©ÓĆ·ÖĄė³öµÄ¹ĢĢåBÅäÖĘ100mL 0.5mol/LµÄČÜŅŗB£¬ĻÖÓŠČēĻĀæɹ©Ń”ŌńµÄŅĒĘ÷£ŗ
A.½ŗĶ·µĪ¹Ü B.ÉÕĘæ C.ÉÕ± D.Ņ©³× E.ĮæĶ² F.ĶŠÅĢĢģĘ½”£
¢ŁÓĆĶŠÅĢĢģĘ½³ĘµĆ¹ĢĢåBµÄÖŹĮæŹĒ_________g”£
¢ŚÅäÖĘČÜŅŗBŹ±£¬ÉĻŹöŅĒĘ÷ÖŠŅ»¶Ø²»ŠčŅŖŹ¹ÓƵÄÓŠ_____________£ØĢī×ÖÄø£©£¬»¹Č±ÉŁµÄŅĒĘ÷ÓŠ__________________________________£ØŠ“ŅĒĘ÷Ćū³Ę£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com