


 �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п���ʵ��ѧ�Ծ��������棩 ���ͣ�ѡ����
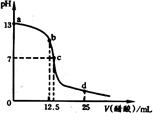
��ͼΪ������0.2 mol/L CH3COOH��Һ��εμӵ�25.00 mL 0.1mol/L NaOH��Һ��pH�ı仯���ߣ�����˵����ȷ���ǣ�

A����ѡ�ü���ָʾCH3COOH�ζ�δ֪Ũ�ȵ�NaOH��Һ �ĵζ��յ㣬��ⶨ���ƫ��
B����Ӧ��������Һ����������������Ŀ��b �����c��
C����ΪCH3COONa��Һ�Լ��ԣ����Ե���CH3COOH��Һ�м���CH3COONaʱ��CH3COOH����̶Ƚ�����
D��a��c�����ڣ�c(Na��)һ������c(CH3COO��)����c(OH��)���ܴ��ڡ�С�ڻ����c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ʡ����ʵ����ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
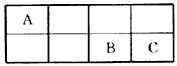
��16�֣���A��B��C���ֶ�����Ԫ�������ڱ������λ������ͼ��
| A | | | |
| | | B | C |

 ����ʽ�� ��
����ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ��Ҧ��ѧ�߶���ѧ�����п���ʵ��ѧ�Ծ����������� ���ͣ���ѡ��
��ͼΪ������0.2 mol/L CH3COOH��Һ��εμӵ�25.00 mL 0.1mol/L NaOH��Һ��pH�ı仯���ߣ�����˵����ȷ���ǣ�
| A����ѡ�ü���ָʾCH3COOH�ζ�δ֪Ũ�ȵ�NaOH��Һ�ĵζ��յ㣬��ⶨ���ƫ�� |
| B����Ӧ��������Һ����������������Ŀ��b �����c�� |
| C����ΪCH3COONa��Һ�Լ��ԣ����Ե���CH3COOH��Һ�м���CH3COONaʱ��CH3COOH����̶Ƚ����� |
| D��a��c�����ڣ�c(Na��)һ������c(CH3COO��)����c(OH��)���ܴ��ڡ�С�ڻ����c(CH3COO��) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com