
”¾ĢāÄæ”æŹµŃéŹŅĄļŠč240mL0.1mol/LµÄCuSO4ČÜŅŗ”£
£Ø1£©ÅäÖĘøĆČÜŅŗŅņŃ”__mLČŻĮæĘ棬ŅŌĻĀÅäÖĘ·½·ØÕżČ·µÄŹĒ__£ØĢī×ÖÄø£©”£
A.³ĘČ”3.84gCuSO4ČÜÓŚŹ¢ÓŠ250mLĖ®µÄÉÕ±ÖŠ
B.³ĘČ”6.0gµØ·ÆČÜÓŚŹ¢ÓŠ250mLĖ®µÄÉÕ±ÖŠ
C.³ĘČ”4.0gCuSO4ČÜÓŚŹ¢ÓŠ250mLĖ®µÄÉÕ±ÖŠ
D.³ĘČ”6.25gµØ·ÆÅä³É250mLČÜŅŗ
£Ø2£©¼ŁČēĘäĖü²Ł×÷¾łÕżČ·ĪŽĪ󣬷ÖĪöĻĀĮŠĒéæö¶ŌÅäÖĘČÜŅŗÅØ¶ČµÄÓ°Ļģ£ØĢī”°Ę«øß”±”°Ę«µĶ”±”°²»±ä”±£©
A.¶ØČŻŹ±ø©ŹÓ¹Ū²ģæĢ¶ČĻߣŗ___£»
B.ŅĘŅŗŹ±£¬¶ŌÓŚČܽāCuSO4(»ņµØ·Æ)µÄÉÕ±Ć»ÓŠĻ“µÓ£ŗ___£»
C.¶ØČŻŗ󣬽«ČŻĮæĘæÕńµ“Ņ”ŌČ£¬¾²ÖĆŹ±·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ÓÖ²¹¼ÓĖ®ÖĮæĢ¶ČĻߣŗ___”£
”¾“š°ø”æ250 D Ę«øß Ę«µĶ Ę«µĶ
”¾½āĪö”æ
£Ø1£©ŹµŃéŹŅŠčÓĆ240mL0.1mol/LµÄĮņĖįĶČÜŅŗ£¬ŅņĪŽ240mL¹ęøńµÄČŻĮæĘ棬¹ŹŠčŃ”ÓĆ250mLµÄČŻĮæĘ棬Źµ¼ŹÉĻÅäÖʵďĒ250mL 0.1mol/LµÄĮņĖįĶČÜŅŗ£¬¾Ż“Ė¶Ōø÷Ń”Ļī½ųŠŠÅŠ¶Ļ£»
£Ø2£©øł¾Żc=![]() æÉµĆ£¬Ņ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗÅäÖʵÄĪó²ī¶¼ŹĒÓÉČÜÖŹµÄĪļÖŹµÄĮænŗĶČÜŅŗµÄĢå»żVŅżĘšµÄ£¬Īó²ī·ÖĪöŹ±£¬¹Ų¼üŅŖæ“ÅäÖĘ¹ż³ĢÖŠŅżĘšnŗĶVŌõŃłµÄ±ä»Æ£ŗČōn±ČĄķĀŪÖµŠ”£¬»ņV±ČĄķĀŪÖµ“óŹ±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«Š”£»Čōn±ČĄķĀŪÖµ“󣬻ņV±ČĄķĀŪÖµŠ”Ź±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«“ó”£
æÉµĆ£¬Ņ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗÅäÖʵÄĪó²ī¶¼ŹĒÓÉČÜÖŹµÄĪļÖŹµÄĮænŗĶČÜŅŗµÄĢå»żVŅżĘšµÄ£¬Īó²ī·ÖĪöŹ±£¬¹Ų¼üŅŖæ“ÅäÖĘ¹ż³ĢÖŠŅżĘšnŗĶVŌõŃłµÄ±ä»Æ£ŗČōn±ČĄķĀŪÖµŠ”£¬»ņV±ČĄķĀŪÖµ“óŹ±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«Š”£»Čōn±ČĄķĀŪÖµ“󣬻ņV±ČĄķĀŪÖµŠ”Ź±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«“ó”£
£Ø1£©ÅäÖĘ240mLČÜŅŗ£¬ŠčŅŖŹ¹ÓĆ250mLČŻĮæĘæÖ»ÄÜÅäÖĘ250mLČÜŅŗ£¬ĮņĖįĶ”¢µØ·ÆµÄČÜŅŗĄļČÜÖŹ¶¼ŹĒCuSO4£¬³ĘČ”ČĪŗĪŅ»ÖÖ¶¼æÉŅŌ£¬ÅäÖĘ250mLČÜŅŗ£¬²¢·ĒŹĒČÜÖŹÖŠ¼ÓČė250mLĖ®£¬A”¢B”¢CĻī“ķĪó£»øł¾ŻÉĻŹö·ÖĪö£¬ČōÅäÖĘ250mLČÜŅŗ£¬ŌņĖłŠčµÄµØ·ÆµÄÖŹĮæm(CuSO45H2O)=0.025mol”Į250gmol-1=6.25g£¬DĻīÕżČ·£»
£Ø2£©A£®¶ØČŻŹ±ø©ŹÓ¹Ū²ģæĢ¶ČĻߣ¬ŅŗĆęŌŚæĢ¶ČĻßĻĀ·½£¬ČÜŅŗµÄĢå»żĘ«Š”£¬ĖłÅäČÜŅŗµÄÅضČĘ«øߣ¬¹Ź“š°øĪŖ£ŗĘ«øߣ»
B£®ŅĘŅŗŹ±£¬Ć»ÓŠĻ“µÓČܽāCuSO4£Ø»ņµØ·Æ£©µÄÉÕ±£¬µ¼ÖĀÅäÖʵÄČÜŅŗÖŠČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ÅäÖʵÄČÜŅŗÅضČĘ«µĶ£¬¹Ź“š°øĪŖ£ŗĘ«µĶ£»
C£®¶ØČŻŗ󣬽«ČŻĮæĘæÕńµ“Ņ”ŌČ£¬¾²ÖĆŹ±·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ÓÖ²¹¼ÓĖ®ÖĮæĢ¶ČĻߣ¬µ¼ÖĀÅäÖʵÄČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹Ź“š°øĪŖ£ŗĘ«µĶ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÅš”¢Į×ŌŖĖŲŌŚ»Æѧ֊ӊŗÜÖŲŅŖµÄµŲĪ»£¬Åš”¢Į×¼°Ęä»ÆŗĻĪļ¹ć·ŗÓ¦ÓĆÓŚæŖ·¢ŠĀŠĶ“¢Ēā²ÄĮĻ”¢³¬µ¼²ÄĮĻ”¢ø»Č¼ĮĻ²ÄĮĻ”¢ø“ŗĻ²ÄĮĻµČøߊĀ²ÄĮĻĮģÓņ”£»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©Ti(BH4)3ŹĒŅ»ÖÖ“¢Ēā²ÄĮĻ£¬æÉÓÉTiCl4ŗĶLiBH4·“Ó¦ÖĘµĆ£®
¢Ł»łĢ¬Ti3+µÄĪ“³É¶Ōµē×ÓŹżÓŠ___øö”£
¢ŚLiBH4ÓÉLi+ŗĶBH4-¹¹³É£¬BH4-³ŹÕżĖÄĆęĢå¹¹ŠĶ”£LiBH4ÖŠ²»“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ___£ØĢī±źŗÅ£©
A£®Ąė×Ó¼ü B£®¹²¼Ū¼ü C£®½šŹō¼ü D£®ÅäĪ»¼ü
£Ø2£©°±ÅšĶé(NH3BH3)ŹĒŅ»ÖÖŠĀŠĶ“¢Ēā²ÄĮĻ£¬Ęä·Ö×ÓÖŠ“ęŌŚÅäĪ»¼ü£¬Ōņ°±ÅšĶé·Ö×Ó½į¹¹Ź½ĪŖ____£¬Š“³öŅ»ÖÖÓė°±ÅšĶ黄ĪŖµČµē×ÓĢåµÄ·Ö×Ó___(Ģī»ÆѧŹ½)”£
£Ø3£©ÅšĖį(H3BO3)ŹĒŅ»ÖÖʬ²ćד½į¹¹°×É«¾§Ģ壬²ćÄŚµÄH3BO3·Ö×Ó¼äĶعżĒā¼üĻąĮ¬[ČēĶ¼]”£

¶ĮĶ¼·ÖĪö1mol H3BO3µÄ¾§ĢåÖŠÓŠ___molĒā¼ü”£
£Ø4£©ĖÄ(Čż±½»łģ¢)īŁ·Ö×Ó½į¹¹ČēĶ¼£ŗ

PŌ×ÓŅŌÕżĖÄĆęĢåµÄŠĪĢ¬Ī§ČĘŌŚīŁŌ×ÓÖŠŠÄÉĻ£¬īŁŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ___£»ÅŠ¶ĻøĆĪļÖŹŌŚĖ®ÖŠ___£ØĢīŠ“”°Ņ×ČÜ”±»ņÕß”°ÄŃČÜ”±£©£¬²¢¼ÓŅŌ½āŹĶ____”£
£Ø£µ£©ÅšĒā»ÆÄĘŹĒŅ»ÖÖ³£ÓƵĻ¹Ō¼Į”£Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾£ŗ

¢ŁøĆ¾§ĢåÖŠNa+µÄÅäĪ»ŹżĪŖ___”£
¢ŚŅŃÖŖÅšĒā»ÆÄĘ¾§ĢåµÄĆܶČĪŖ¦Ńg/cm3£¬NA“ś±ķ°¢·üŁ¤µĀĀŽ³£ŹżµÄÖµ£¬Ōņa=___(ÓĆŗ¬¦Ń”¢NAµÄ×ī¼ņŹ½×Ó±ķŹ¾)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĀČĖįŹĒŅ»ÖÖĒæĖį£¬ÅØ¶Č³¬¹ż40£„Ź±»į·¢Éś·Ö½ā£¬·“Ó¦æɱķŹ¾ĪŖ£ŗa HClO3 = bO2”ü+ c Cl2”ü+ d HClO4 + e H2O”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ ( )
A. ÓÉ·“Ó¦æÉČ·¶Ø£ŗŃõ»Æ²śĪļŹĒHClO4
B. ÓÉ·Ē½šŹōŠŌCl£¾S£¬æÉĶĘÖŖĖįŠŌHClO3£¾H2SO4
C. Čō»Æѧ¼ĘĮæŹża=8£¬b=3£¬ŌņøĆ·“Ó¦×ŖŅʵē×ÓŹżĪŖ8e£
D. ČōøĆ·“Ó¦ĖłµĆlmol»ģŗĻĘųĢåÖŹĮæĪŖ45g£¬Ōņ·“Ó¦æɱķŹ¾ĪŖ£ŗ3HClO3 = 2O2”ü+ C12”ü+ HClO4 + H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©Ń”ŌńĻĀĮŠŹµŃé·½·Ø·ÖĄėĪļÖŹ£¬½«·ÖĄė·½·ØµÄŠņŗÅĢīŌŚŗįĻßÉĻ£ŗa”¢ŻĶČ”·ÖŅŗ·Ø£»b”¢¼ÓČČ·Ö½ā£» c”¢½į¾§·Ø£» d”¢·ÖŅŗ·Ø£» e”¢ÕōĮó·Ø£» f”¢¹żĀĖ·Ø£» g”¢ÉųĪö·Ø
¢Ł_____“ÓĻõĖį¼ŲŗĶĀČ»ÆÄʵĻģŗĻČÜŅŗÖŠ»ńµĆĻõĖį¼Ų
¢Ś______·ÖĄėĖ®ŗĶ±½µÄ»ģŗĻĪļ
¢Ū_____³żČ„ĒāŃõ»ÆĢś½ŗĢåÖŠ»ģÓŠµÄNaCl
¢Ü______·ÖĄėµāŗĶĖÄĀČ»ÆĢ¼
£Ø2£©ŹµŃéŹŅÓĆ4.0 mol/L NaClČÜŅŗÅäÖĘ100mL 0.50 mol/L NaClČÜŅŗ”£
¢ŁŹµŃéŠčŅŖ²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢ÉÕ±”¢ĮæĶ²Ķā£¬»¹ŠčŅŖµÄ²£Į§ŅĒĘ÷ŹĒ____________”£
¢ŚÅäÖĘÉĻŹöČÜŅŗ£¬ŠčŅŖ4.0 mol/L NaClČÜŅŗ_________mL”£
¢ŪÅäÖĘ¹ż³ĢÖŠ³öĻÖŅŌĻĀĒéæö£¬Ź¹ĖłÅäČÜŅŗÅضČĘ«µĶ_____________”£
A£®ČÜŅŗ×ŖŅĘŗóƻӊĻ“µÓÉÕ±ŗĶ²£Į§°ō”£ B£®¶ØČŻŹ±ŃöŹÓČŻĮæĘæµÄæĢ¶ČĻß”£
C. Ī“ĄäČ“¼“½ųŠŠ×ŖŅĘ”¢¶ØČŻ²Ł×÷”£ D£®ČŻĮæĘæÖŠŌÓŠÉŁĮæÕōĮóĖ®
E£®Čē¹ū¼ÓĖ®³¬¹żĮĖæĢ¶ČĻߣ¬Č”³öČÜŅŗŹ¹ŅŗĆęĒ”ŗƵ½æĢ¶ČĻß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·“Ó¦ÖŠ£¬²»ŹōÓŚŃõ»Æ»¹Ō·“Ó¦µÄŹĒ£Ø £©
A.H2+Cl2=2HCl
B.2KMnO4![]() K2MnO4+MnO2+O2ӟ
K2MnO4+MnO2+O2ӟ
C.NH4HCO3![]() NH3ӟ+CO2ӟ+H2O
NH3ӟ+CO2ӟ+H2O
D.Zn+H2SO4=H2ӟ+ZnSO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆSCR¼¼ŹõæÉÓŠŠ§½µµĶ²ńÓĶ·¢¶Æ»śNOxÅÅ·Å”£SCR¹¤×÷ŌĄķĪŖÄņĖŲ[CO(NH2)2]Ė®ČÜŅŗČČ·Ö½āĪŖNH3ŗĶCO2£¬ŌŁĄūÓĆNH3×Ŗ»ÆNOx£¬×°ÖĆČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©

A.×Ŗ»ÆNO2¹ż³ĢµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ8NH3+6NO2![]() 7N2+12H2O
7N2+12H2O
B.×Ŗ»ÆĘ÷¹¤×÷¹ż³ĢÖŠ£¬µ±×ŖŅĘ0.6molµē×ÓŹ±£¬»įĻūŗÄ4.48LNH3
C.ÄņĖŲĖ®ČÜŅŗČČ·Ö½ā·“Ó¦²»ŹōÓŚŃõ»Æ»¹Ō·“Ó¦
D.øĆ×°ÖĆ×Ŗ»ÆNOŹ±£¬»¹Ō¼ĮÓėŃõ»Æ¼ĮĪļÖŹµÄĮæÖ®±ČĪŖ2:3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚŅ»¶ØĢå»żµÄŗ¬ÓŠAl3+”¢Mg2+”¢Ba2+µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČėNaOHŗĶNa2SO4µÄ»ģŗĻČÜŅŗ£Ø³żÉĻŹöĄė×ÓĶā£¬ĘäĖūĄė×Ó²»ÓėĖł¼ÓŹŌ¼Į·“Ó¦£¬¼ŁÉčAl3+”¢Mg2+½įŗĻOH-µÄÄÜĮ¦ĻąĶ¬£©£¬²śÉś³ĮµķµÄĪļÖŹµÄĮæÓėĖł¼ÓČÜŅŗµÄĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£ŗ
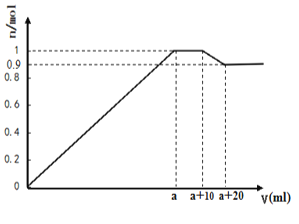
A. µ±a<V<a+10Ź±£¬²»ŌŁÉś³ÉÄŃČÜĪļ
B. ÉĻŹöŗ¬Al3+”¢Mg2+”¢Ba2+µÄ»ģŗĻČÜŅŗÖŠ3c(Al3+)+2c(Mg2+)=c(Ba2+)
C. ÉĻŹöŗ¬Al3+”¢Mg2+”¢Ba2+µÄ»ģŗĻČÜŅŗÖŠn(Al3+) = 0.2mol
D. NaOHŗĶNa2SO4µÄ»ģŗĻČÜŅŗÖŠc(NaOH)>c(Na2SO4)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫1.12 gĢś·Ū¼ÓČė25 mL 2 mol”¤L£1µÄĀČ»ÆĢśČÜŅŗÖŠ£¬³ä·Ö·“Ó¦ŗó£¬Ęä½į¹ūŹĒ
A. Ģś·ŪÓŠŹ£Óą£¬ČÜŅŗ³ŹĒ³ĀĢÉ«£¬Cl£»ł±¾±£³Ö²»±äB. Fe2£«ŗĶFe3£«ĪļÖŹµÄĮæÖ®±ČĪŖ5”Ć1
C. ĶłČÜŅŗÖŠµĪČėĪŽÉ«KSCNČÜŅŗ£¬ĻŌŗģÉ«D. Ńõ»Æ²śĪļŗĶ»¹Ō²śĪļµÄĪļÖŹµÄĮæÖ®±ČĪŖ2”Ć5
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ°“ŅŖĒóĢīæÕ
(1)1mol Na2O2¹ĢĢåÓėĖ®ĶźČ«·“Ó¦Ź±×ŖŅʵĵē×ÓŹż_____________£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________”£
(2)¹¤ŅµÉĻÓÉ»ŌĶæóÉś²śĶµÄÖ÷ŅŖ·“Ó¦ĪŖ£ŗCu2S+O2![]() 2Cu+SO2£¬øĆ·“Ó¦ÖŠ±»»¹ŌµÄŌŖĖŲŹĒ__________(ĢīŌŖĖŲ·ūŗÅ)”£
2Cu+SO2£¬øĆ·“Ó¦ÖŠ±»»¹ŌµÄŌŖĖŲŹĒ__________(ĢīŌŖĖŲ·ūŗÅ)”£
(3)·“Ó¦(2)ÖŠ²śÉśµÄSO2Ī²ĘųæÉÓĆNaOHČÜŅŗĪüŹÕ£¬ČōÓĆ1L 1mol/LµÄNaOHČÜŅŗĪüŹÕ±ź×¼×“æöĻĀ22.4L SO2£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com