
XY2(l)+3O2(g)=XO2(g)+2YO2(g)”£ĄäČ“ŗó£¬ŌŚ±ź×¼×“æöĻĀ²āµĆÉś³ÉĪļµÄĢå»żŹĒ672 mL£¬ĆܶȏĒ
(1)·“Ó¦Ē°O2µÄĢå»żŹĒ__
(2)»ÆŗĻĪļXY2µÄĦ¶ūÖŹĮæŹĒ__
(3)ČōXY2·Ö×ÓÖŠX”¢YĮ½ŌŖĖŲµÄÖŹĮæ±ČŹĒ3”Ć16£¬ŌņX”¢YĮ½ŌŖĖŲ·Ö±šĪŖ________ŗĶ________”££ØĢīŌŖĖŲ·ūŗÅ£©?
½āĪö£ŗøł¾ŻĢāÄæÖŠµÄ»Æѧ·½³ĢŹ½ÖŖøĆ·“Ó¦ĪŖ·“Ó¦Ē°ŗóĘųĢåĢå»ż²»±äµÄ·“Ó¦£¬¹Ź·“Ó¦Ē°ŃõĘųµÄĢå»żµČÓŚ·“Ó¦ŗóĘųĢåµÄĢå»ż£¬¼“672 mL£¬ÓÖ·“Ó¦ŗóĖłµĆĘųĢåµÄÖŹĮæĪŖ
“š°ø£ŗ£Ø1£©672 mL””£Ø2£©



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØÖŹĮæµÄŅŗĢ¬»ÆŗĻĪļXY2£¬ŌŚŅ»¶ØÖŹĮæµÄO2ÖŠĒ”ŗĆĶźČ«Č¼ÉÕ£¬»Æѧ·½³ĢŹ½ĪŖXY2(l)+3O2(g)=XO2(g)+2YO2(g)£¬ĄäČ“ŗó£¬ŌŚ±ź×¼×“æöĻĀ²āµĆÉś³ÉĪļµÄĢå»żŹĒ672 mL,ĆܶȏĒ2.56 g/L”£
(1)·“Ó¦Ē°O2µÄĢå»żŹĒ mL”£
(2)»ÆŗĻĪļXY2µÄĦ¶ūÖŹĮæŹĒ g/mol”£
(3)ČōXY2·Ö×ÓÖŠX”¢YĮ½ŌŖĖŲµÄÖŹĮæ±ČŹĒ3”Ć16£¬ŌņX”¢YĮ½ŌŖĖŲ·Ö±šĪŖ ŗĶ ”£(Š“ŌŖĖŲ·ūŗÅ)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØÖŹĮæµÄŅŗĢ¬»ÆŗĻĪļXY2£¬ŌŚŅ»¶ØĮæµÄO2ÖŠĒ”ŗĆĶźČ«Č¼ÉÕ£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗXY2£ØŅŗ£©+3O2(Ęų)=XO2£ØĘų£©+2YO2£ØĘų£©”£ĄäČ“ŗó£¬ŌŚ±ź×¼×“æöĻĀ²āµĆÉś³ÉĪļµÄĢå»żŹĒ672mL£¬ĆܶȏĒ2.56g/L£¬Ōņ£ŗ
£Ø1£©·“Ó¦Ē°O2Ģå»żŹĒ ”£ £Ø2£©»ÆŗĻĪļXY2µÄĦ¶ūÖŹĮæŹĒ ”£
£Ø3£©ČōXY2·Ö×ÓÖŠX”¢YĮ½ÖÖŌŖĖŲµÄÖŹĮæ±ČŹĒ3”Ć16 ”£ŌņX”¢YĮ½ÖÖŌŖĖŲ·Ö±šĪŖ
ŗĶ ”££ØŠ“ŌŖĖŲ·ūŗÅ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½Ī÷Ź”ÉĻČÄŹŠøßČżµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā¹²12·Ö£©
¢ń”¢µŖŌŖĖŲµÄĒā»ÆĪļŗĶŃõ»ÆĪļŌŚ¹¤ŅµÉś²śŗĶ¹ś·Ą½ØÉčÖŠ¶¼ÓŠ¹ć·ŗÓ¦ÓĆ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)µŖŌŖĖŲŌ×ÓµÄL²ćµē×ÓŹżĪŖ £»
(2) NH3ÓėNaClO·“Ó¦æɵƵ½ėĀ(N2H4)£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
(3)ėĀæÉ×÷ĪŖ»š¼ż·¢¶Æ»śµÄČ¼ĮĻ£¬ÓėŃõ»Æ¼ĮN2O4·“Ӧɜ³ÉN2ŗĶĖ®ÕōĘų”£
ŅŃÖŖ£ŗ¢ŁN2(g)+2O2(g) = N2O4(l) ¦¤H1=-19.5kJ∙mol£1
¢ŚN2H4(l) + O2 (g)=N2(g)+2H2O(g) ¦¤H2 =-534.2 kJ”¤mol£1
Š“³öėĀŗĶN2O4·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ £»
(4)ėĀŅ»æÕĘųČ¼ĮĻµē³ŲŹĒŅ»ÖÖ¼īŠŌµē³Ų£¬øƵē³Ų·ÅµēŹ±£¬øŗ¼«µÄ·“Ó¦Ź½ĪŖ ”£
¢ņ”¢Ņ»¶ØÖŹĮæµÄŅŗĢ¬»ÆŗĻĪļ ŌŚ±ź×¼×“æöĻĀµÄŅ»¶ØÖŹĮæµÄ
ŌŚ±ź×¼×“æöĻĀµÄŅ»¶ØÖŹĮæµÄ ÖŠĒ”ŗĆĶźČ«Č¼ÉÕ,·“Ó¦·½³ĢŹ½ĪŖ:
ÖŠĒ”ŗĆĶźČ«Č¼ÉÕ,·“Ó¦·½³ĢŹ½ĪŖ: 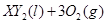 ===
===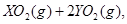 ĄäČ“ŗó,ŌŚ±ź×¼×“æöĻĀ²āµĆÉś³ÉĪļµÄĢå»żŹĒ672 mL,ĆܶȏĒ2.56
ĄäČ“ŗó,ŌŚ±ź×¼×“æöĻĀ²āµĆÉś³ÉĪļµÄĢå»żŹĒ672 mL,ĆܶȏĒ2.56  Ōņ:
Ōņ:
(1)·“Ó¦Ē° µÄĢå»żŹĒ
ӣ
(2)»ÆŗĻĪļ
µÄĢå»żŹĒ
ӣ
(2)»ÆŗĻĪļ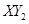 µÄĦ¶ūÖŹĮæŹĒ
ӣ
µÄĦ¶ūÖŹĮæŹĒ
ӣ
(3)Čō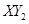 ·Ö×ÓÖŠX”¢YĮ½ŌŖĖŲµÄÖŹĮæ±ČŹĒ3”Ć16,ŌņX”¢YĮ½ŌŖĖŲ·Ö±šĪŖ
ŗĶ
(Š“ŌŖĖŲ·ūŗÅ)”£
·Ö×ÓÖŠX”¢YĮ½ŌŖĖŲµÄÖŹĮæ±ČŹĒ3”Ć16,ŌņX”¢YĮ½ŌŖĖŲ·Ö±šĪŖ
ŗĶ
(Š“ŌŖĖŲ·ūŗÅ)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com