
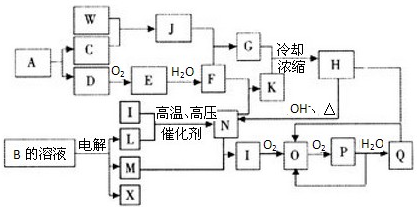
��ͼ��һЩ���ʼ��ת����ϵͼ��ͼ���������ʺ��е�Ԫ����ֻ��һ�ֲ��Ƕ�����Ԫ�أ����з�Ӧ������P��Һ�е�ˮ�Լ����ַ�Ӧ��һЩ������δ�����C��D��E��KΪ���ʣ�NΪ���Ի����J��������Ԫ����ɵĺ�SiC��ͬ�������͵����Ͳ��ϣ���J��SiC������ͬ�ļ۵�������ԭ������I��������Ԫ����ɵ�ǿ���Ӧ�����ڼ���һ�ֳ������л������ţ���Ӧ�ݡ������ڹ�ҵ������B��

�ش��������⣺
��1��д���������ʵĻ�ѧʽ��G ��I ��
��2��д����Ӧ�١��ߵ����ӷ���ʽ��
�� ��
�� ��
��3��J��SiC���������Ϸ�ĩ����һ�����ģ���ɵ�·�����ɢ�Ȳ��ϡ���Ӧ�����ձ���ѧ���о������Ʊ����������Ϸ�ĩ������;������֪��Ӧ����n(F)��n(E)��n(J)��n(SiC)��n(K)��1��2��4��1��3��д����Ӧ�ڵĻ�ѧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α��һ��(����1)��2009��2010ѧ�ꡡ��19��26�ڡ��ܵ�175��182�� �˽̿α�� ���ͣ�022
��ͼ��ʾһЩ����֮���ת����ϵ��ÿ����ĸ����һ�����ʣ����ֲ�������ȥ������
A��һ����ʽ�Σ�Ҳ��һ�ֳ��õĻ�ѧ���ϣ�B��һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壬D��һ����ɫ��ζ�����壮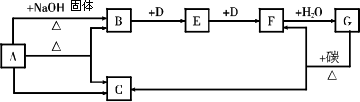
(1)����A��������________��
(2)����B��һ����Ҫ��;��________��
(3)ʵ������ȡ����B�Ļ�ѧ����ʽ��________����ҵ��ȡ����B�Ļ�ѧ����ʽ��________��
(4)д���������ʼ�ת���Ļ�ѧ����ʽ��
��B��E��________��
��F��G��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
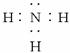
��1��д��X������_____________��A�ĵ���ʽ_____________��
��2��д��AD��Ӧ�Ļ�ѧ����ʽ___________________________________________��
��3��д��C��������ʱ�Ļ�ѧ����ʽ����дһ�֣�_________________________________��
��4��д��N��ϡ��Һ�����M��Ӧ�����ӷ���ʽ___________________________________��
��5��0.3 mol M������B��Ӧת�Ƶ��ӵ����ʵ���Ϊ____________mol��
��6�������Ľ�ʹ��ҩ���������������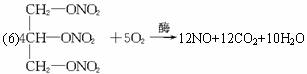 ����������Һ��ø�������£����������ͷų�D���ӣ�ͬʱ���ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________��
����������Һ��ø�������£����������ͷų�D���ӣ�ͬʱ���ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com