
�ס��ҡ�������������������ڵ����ʼ�������ͼ��ʾ����������������ݻ���ȣ��¶���ͬ����Ӧ�мס������ݻ����䣬���е�ѹǿ���䣬��һ���¶��·�Ӧ�ﵽƽ�⡣����˵����ȷ����
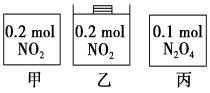
A��ƽ��ʱ��������c��NO2���Ĵ�С˳��Ϊ��>��>��
B��ƽ��ʱN2O4�İٷֺ�������>�ף���
C��ƽ��ʱ����NO2�����N2O4��ת������ͬ
D��ƽ��ʱ������ƽ����Է�����������>��>��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶��ϵڶ��ο��Ի�ѧ���������棩 ���ͣ������
( 14��)
I .��1����֪H2��ȼ����285.8KJ/mol��д��Һ̬ˮ�������H2��O2���Ȼ�ѧ����ʽ ��
��2����֪2SO2(g)+O2(g) = 2SO3(g) ��H����197 kJ/mol����ͬ�¶Ⱥ�ѹǿ�����£�4molSO2��2molO2���������з�Ӧ��ƽ��ʱ�ų�������ΪQ KJ����Q 394KJ(�>����<����=��)
II.�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI��CO2(g)+3H2(g) CH3OH
CH3OH (g)+H2O(g) ��H1
(g)+H2O(g) ��H1
��ӦII��CO(g)+2H2(g) CH3OH(g) ��H2
CH3OH(g) ��H2
�±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)
�¶� | 250�� | 300�� | 350�� |
K | 2.041 | 0.270 | 0.012 |
��1���ɱ��������жϦ�H2 0(�����������������)��
��2���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� ��
A�������¶�
B����CH3OH(g)����ϵ�з���
C��ʹ�ú��ʵĴ���
D�����º��ݳ���He��ʹ��ϵ��ѹǿ����
E����ԭ�����ٳ��� CO�� H2
��3��ij�¶��£���2 mol CO��6 mol H2����2L�ĺ����ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)=0.2 mol��L-1����CO��ת����Ϊ ����ʱ���¶�Ϊ (���ϱ���ѡ��)��
��4�������£�1mol CO��nmol H2��һ���ݻ��ɱ���ܱ������з�Ӧ�ﵽƽ�������a molCH3OH������ʼʱ����3molCO+3nmolH2�����ƽ��ʱ����CH3OH_______mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʦ���и������¿��Ļ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧС���ͬѧģ�ҵ���������������ͼ��ʾ��װ�á���֪��
CaCl2��nH2O��CaCl2��nH2O��CaCl2��8NH3��[Ca(NH3)8]Cl2�������������������գ�

��1����Һ©���а�ˮ��Ũ��Ϊ9.0 mol/L��������������Ϊ35%���ܶ�Ϊ0.88 g/cm3�İ�ˮ����9.0 mol/L�İ�ˮ100 mL����Ҫ�Ķ��������� (ѡ����)��
a��100 mL����ƿ b��10 mL��Ͳ c��50 mL��Ͳ d��������ƽ
��2������ʱ�����з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ�鿪ʼ�ȼ��ȴ����������������ʱ�ٴ�Һ©�����������߾ƾ��ƣ��ɹ۲쵽�������� ��
��4������ܼ������� ������ʢ�ŵ�ҩƷΪ (ѡ�����б��)����Ŀ���� ��
a��ŨH2SO4 b����ˮCaCl2 c����ʯ�� d����ˮCuSO4
��5�����г���NO֮�⣬�����ܴ��ڵ������� (��д��ѧʽ)���ձ��з�����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������ĵ������һ����ѧ����12���¿���ѧ���������棩 ���ͣ�ʵ����
����һ�ݺ���FeCl3��FeCl2�������Ϊ�ⶨ���ɷֵĺ���������������ʵ�飺
ʵ��1���� ��ȡһ�� ��������Ʒ������Ʒ�ܽ⣻
��������Ʒ������Ʒ�ܽ⣻
�� ���ܽ�����Һ�м���������AgNO3��Һ������������
�� ���������ˡ�ϴ�ӡ�����õ���ɫ����17.22 g��
ʵ��2���� ��ȡ��ʵ��1����ͬ��������Ʒ������Ʒ�ܽ⣻
�� ���ܽ�����Һ��ͨ��������Cl2��
�� �����������Һ�м���������NaOH��Һ���õ����ɫ������
�� ���������ˡ�ϴ�Ӻ������գ����������ټ��٣��õ���������4g��
�ش��������⣺
��1���ܽ���������õ��IJ���������___________________��
��2��ʵ���ұ���FeCl2��Һʱͨ���������м��������Լ�_____________��_____________��
��3��ʵ��2ͨ������Cl2��Ŀ����________ ��
�漰�Ļ�ѧ��Ӧ�����ӷ���ʽ��______ _��
��4����μ����ܽ���Ʒ�к���Fe2+ ��
��5��ͨ��ʵ���������ݣ����������Ʒ��FeCl3��FeCl2�����ʵ���֮��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������ĵ������һ����ѧ����12���¿���ѧ���������棩 ���ͣ�ѡ����
��2L�ܱ������н��з�ӦC(s)+H2O(g) CO(g)+H2(g)��H>0�����c(H2O)�淴Ӧʱ��(t)�ı仯��ͼ�������ж���ȷ����
CO(g)+H2(g)��H>0�����c(H2O)�淴Ӧʱ��(t)�ı仯��ͼ�������ж���ȷ����
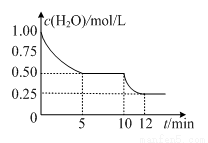
A��5minʱ�÷�Ӧ��Kֵһ��С��12 minʱ��Kֵ
B��0~5min�ڣ�v (H2)=0.05mol/(L•min)
C��10 minʱ���ı��������������Ǽ�Сѹǿ
D���÷�Ӧ��������ƽ����Է���������5minʱС��12 min ʱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������ĵ������һ����ѧ����12���¿���ѧ���������棩 ���ͣ�ѡ����
ij���᳧������SO2�Ļ������÷�������ͼ��ʾ������˵������ȷ����

A��X���ܺ���2���� B��a��SO3
C��Y�к��У�NH4��2SO4 D����NH4��2S2O8��S�Ļ��ϼ۲�����Ϊ+7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������ĵ������һ����ѧ����12���¿���ѧ���������棩 ���ͣ�ѡ����
ijѧ������˿��Cl2Ϊԭ�Ͻ�����������ʵ�顣�ӷ���Ƕȷ���������ѡ����ȷ����
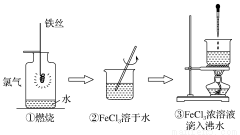
A��ʵ��١������漰�����ʾ�Ϊ�����
B��ʵ��ڡ��۾�Ϊ���ȷ�Ӧ
C��ʵ��ڡ��۾�δ����������ԭ��Ӧ
D��ʵ��١��۷�Ӧ�Ƶõ����ʾ�Ϊ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017����������������и��������տ�������ѧ���������棩 ���ͣ�ѡ����
25��ʱ������˵������ȷ����
A�����ʵ���Ũ����ȵ�����Ͱ�ˮ�������ϣ�������Һ��pH<7
B�����ʵ���Ũ����ȵ�����Ͱ�ˮ�������ϣ�������Һ��pH<7
C��pH=3�������pH=11�İ�ˮ�������ϣ�������Һ��pH>7
D��pH=3�������pH=11�İ�ˮ�������ϣ�������Һ��pH<7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ������
������ʱ��pH=a�İ�ˮ��PH =b������������ϣ�ǡ����ȫ��Ӧ����ˮ����ȿɱ�ʾΪ_______________ (�ðٷ�������ʾ��
��2�������£�0.1 mol/LNaClO��Һ��pH_________0.1 mol/LNa2SO3��Һ��pH����ѡ����ڡ�����С�ڡ������ڡ�)��Ũ�Ⱦ�Ϊ0.1mol/L��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ ��
��֪��
H2SO3 | Kal=1.54��l0-2 | Ka2=1.02��10-7 |
HCIO | Ka1=2.95��10-8 | |
H2CO3 | Kal=4.3��10-7 | Ka2=5.6��10-11 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com