
| A��������ˮϴ���ձ���������2-3�Σ�ϴ��Һ��ע������ƿ���� |
| B����������ƽ���������Na2CO3��10H2O���壬�����ձ��У��ټ�������ˮ���ò���������������ʹ����ȫ�ܽ� |
| C��������ȴ��Na2CO3��Һ�ز�����ע������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
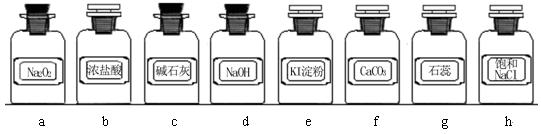
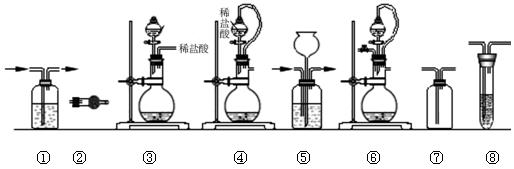
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �ۡ��� | �� | ��/g | �� |
| C | �� | �� | ��/e | �� |
| D | �ܡ��� | �� | ��/g | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
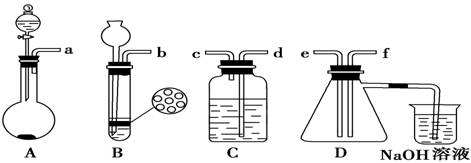
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ɷ� | ������g�� | Ħ��������g��mol��1�� |
| ���� | 50.0 | 342 |
| ����� | 0.5 | 174 |
| ��˾ƥ�� | 0.4 | 180 |
| ������� | 0.5 | 158 |
| ������ | 0.2 | 170 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
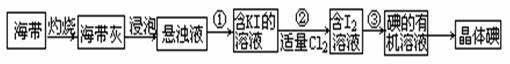

| A�����Ȼ�̼ | B������ | C���ƾ� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʳ�� | B������FeCl3��Һ | C������ | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
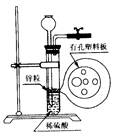
| A���٢ڢ� | B���ڢ� |
| C���ڢۢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com