
A”¢B”¢C”¢DŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°36ŗÅŌŖĖŲ,ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó”£AŌ×ÓL²ćµÄ³É¶Ōµē×ÓŹżŗĶĪ“³É¶Ōµē×ÓŹżĻąµČ,BŌ×ÓµÄ×īĶā²ćp¹ģµĄµÄµē×ÓĪŖ°ė³äĀś½į¹¹,CŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ”£DŹĒµŚĖÄÖÜĘŚŌŖĖŲ,ĘäŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹżÓėĒāŌ×ÓĻąĶ¬,ĘäÓąø÷²ćµē×Ó¾ł³äĀś”£Ēė»Ų“šĻĀĮŠĪŹĢā:
(1)A”¢B”¢CµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņŹĒ””””””””(ÓƶŌÓ¦µÄŌŖĖŲ·ūŗűķŹ¾);»łĢ¬DŌ×ӵĵē×ÓÅŲ¼Ź½ĪŖ”””””””””””””””””£
(2)AµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ·Ö×ÓÖŠ,ĘäÖŠŠÄŌ×Ó²ÉČ”””””””””ŌÓ»Æ;B µÄæռ乹ŠĶĪŖ””””””””(ÓĆĪÄ×ÖĆčŹö)”£
µÄæռ乹ŠĶĪŖ””””””””(ÓĆĪÄ×ÖĆčŹö)”£
(3)1 mol AB-ÖŠŗ¬ÓŠµÄ¦Š¼üøöŹżĪŖ”””””””””£
(4)ČēĶ¼ŹĒ½šŹōCaŗĶDĖłŠĪ³ÉµÄijÖÖŗĻ½šµÄ¾§°ū½į¹¹Ź¾ŅāĶ¼,ŌņøĆŗĻ½šÖŠCaŗĶDµÄŌ×ÓøöŹż±ČŹĒ”””””””””£
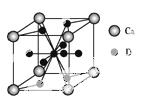
(5)ļēÄųŗĻ½šÓėÉĻŹöŗĻ½š¶¼¾ßÓŠĻąĶ¬ĄąŠĶµÄ¾§°ū½į¹¹XYn,ĖüĆĒÓŠŗÜĒæµÄ“¢ĒāÄÜĮ¦”£ŅŃÖŖļēÄųŗĻ½šLaNin¾§°ūĢå»żĪŖ9.0”Į10- 23 cm3,“¢ĒāŗóŠĪ³ÉLaNinH4.5ŗĻ½š(Ēā½ųČė¾§°ūæÕĻ¶,Ģå»ż²»±ä),ŌņLaNinÖŠn=””””””””(ĢīŹżÖµ);ĒāŌŚŗĻ½šÖŠµÄĆܶČĪŖ”””””””””£
23 cm3,“¢ĒāŗóŠĪ³ÉLaNinH4.5ŗĻ½š(Ēā½ųČė¾§°ūæÕĻ¶,Ģå»ż²»±ä),ŌņLaNinÖŠn=””””””””(ĢīŹżÖµ);ĒāŌŚŗĻ½šÖŠµÄĆܶČĪŖ”””””””””£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÕżČ·ÕĘĪÕ»ÆѧÓĆÓļŗĶ»Æѧ»ł±¾øÅÄīŹĒѧŗĆ»ÆѧµÄ»ł“””£ĻĀĮŠÓŠ¹Ų±ķŹöÖŠÕżČ·µÄŅ»×éŹĒ
A£®16OÓė18O»„ĪŖĶ¬Ī»ĖŲ£»H216O”¢D216O”¢H218O”¢D218O»„ĪŖĶ¬ĖŲŅģŠĪĢå
B£®SiH4”¢PH3”¢HClµÄĪČ¶ØŠŌÖš½„ŌöĒæ
C£®¹żŃõŅŅĖį(CH3COOO H)ÓėōĒ»łŅŅĖį(HOCH2COOH)Ėłŗ¬¹ŁÄÜĶÅĻąĶ¬£»Į½Õß»„ĪŖĶ¬·ÖŅģ ¹¹Ģå
H)ÓėōĒ»łŅŅĖį(HOCH2COOH)Ėłŗ¬¹ŁÄÜĶÅĻąĶ¬£»Į½Õß»„ĪŖĶ¬·ÖŅģ ¹¹Ģå
D£®Ca2+µÄ½į¹¹Ź¾ŅāĶ¼ĪŖ£¬ NH4ClµÄµē×ÓŹ½ĪŖ
NH4ClµÄµē×ÓŹ½ĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«ŗ£Ė®µ»ÆÓėÅØŗ£Ė®×ŹŌ“»Æ½įŗĻĘšĄ“ŹĒ×ŪŗĻĄūÓĆŗ£Ė®µÄÖŲŅŖĶ¾¾¶Ö®Ņ»”£Ņ»°ćŹĒĻČ½«ŗ£Ė®µ»Æ»ńµĆµĖ®£¬ŌŁ“ÓŹ£ÓąµÄÅØŗ£Ė®ÖŠĶعżŅ»ĻµĮŠ¹¤ŅÕĢįČ”ĘäĖū²śĘ·”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĻĀĮŠøĽųŗĶÓÅ»Æŗ£Ė®×ŪŗĻĄūÓĆ¹¤ŅÕµÄÉčĻėŗĶ×ö·ØæÉŠŠµÄŹĒ £ØĢīŠņŗÅ£©”£
¢ŁÓĆ»ģÄż·Ø»ńČ”µĖ® ¢ŚĢįøß²æ·Ö²śĘ·µÄÖŹĮæ
¢ŪÓÅ»ÆĢįČ”²śĘ·µÄĘ·ÖÖ ¢ÜøĽų¼Ų”¢äå”¢Ć¾µÄĢįČ”¹¤ŅÕ
£Ø2£©²ÉÓĆ”°æÕĘų“µ³ö·Ø”±“ÓÅØŗ£Ė®ÖŠ“µ³öBr2£¬²¢ÓĆ“æ¼īĪüŹÕ”£¼īĪüŹÕäåµÄÖ÷ŅŖ·“Ó¦ŹĒBr2+Na2CO3+H2O 
 NaBr + N
NaBr + N aBrO3+NaHCO3£¬ĪüŹÕ1mol Br2Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ mol”£
aBrO3+NaHCO3£¬ĪüŹÕ1mol Br2Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ mol”£
£Ø3£©ŗ£Ė®ĢįĆ¾µÄŅ»¶Ī¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ
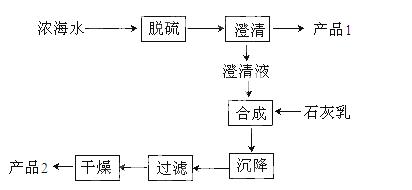
ÅØŗ£Ė®µÄÖ÷ŅŖ³É·ÖČēĻĀ£ŗ
| Ąė×Ó | N | Mg2+ | Cl- | SO42- |
| ÅضČ/g/L | 63.7 | 28.8 | 144.6 | 46.4 |
øĆ¹¤ŅÕ¹ż³ĢÖŠ£¬ĶŃĮņ½×¶ĪÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬²śĘ·2µÄ»ÆѧŹ½ĪŖ £¬1LÅØŗ£Ė®×ī¶ąæɵƵ½²ś Ę·2µÄÖŹĮæĪŖ g”£
Ę·2µÄÖŹĮæĪŖ g”£
£Ø4£©²ÉÓĆŹÆÄ«Ńō¼«”¢²»ŠāøÖŅõ¼«µē½āČŪČŚµÄĀČ»ÆĆ¾£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»µē½āŹ±£¬ČōÓŠÉŁĮæĖ®“ęŌŚ»įŌģ³É²śĘ·Ć¾µÄĻūŗÄ£¬Š“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²Īæ¼ĻĀ±ķÖŠĪļÖŹµÄČŪµć£¬»Ų“šÓŠ¹Ų ĪŹĢā£ŗ
ĪŹĢā£ŗ
| ĪļÖŹ | NaF | NaCl | NaBr | NaI | NaCl | KCl | RbCl | CsCl |
| ČŪµć/”ę | 995 | 801 | 755 | 651 | 801 | 776 | 715 | 646 |
| ĪļÖŹ | SiF4 | SiCl4 | SiBr4 | SiI4 | SiCl4 | GeCl4 | SnCl4 | PbCl4 |
| ČŪµć/”ę | -90.4 | £70.4 | 5.2 | 120 | £70.4 | £49.5 | £36.2 | £15 |
(1)ÄʵÄĀ±»ÆĪļ¼°¼ī½šŹōµÄĀČ»ÆĪļµÄČŪµćÓėĀ±ĖŲĄė×Ó¼°¼ī½šŹōĄė×ÓµÄ________ÓŠ¹Ų£¬Ėę×Å________µÄŌö“ó£¬ČŪµćŅĄ“Ī½µµĶ”£
(2)¹čµÄĀ±»ÆĪļµÄČŪµć¼°¹č”¢Õą”¢Īż”¢Ē¦µÄĀČ»ÆĪļµÄČŪµćÓė________ÓŠ¹Ų£¬Ėę×Å________Ōö“ó£¬________Ōö“󣬹ŹČŪµćŅĄ“ĪÉżøß”£
(3)ÄʵÄĀ±»ÆĪļµÄČŪµć±ČĻąÓ¦µÄ¹čµÄĀ±»ÆĪļµÄČŪµćøߵƶą£¬ÕāÓė________ÓŠ¹Ų£¬ŅņĪŖ_______________£¬
¹ŹĒ°ÕßµÄČŪµćŌ¶øßÓŚŗóÕß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µŖ»ÆÅš(BN)¾§ĢåÓŠ¶ąÖÖĻą½į¹¹”£Įł·½ĻąµŖ»ÆÅšŹĒĶس£“ęŌŚµÄĪȶØĻą£¬ÓėŹÆÄ«ĻąĖĘ£¬¾ßÓŠ²ćד½į¹¹£¬æÉ×÷øßĪĀČ󻬼Į”£Į¢·½ĻąµŖ»ÆÅšŹĒ³¬Ó²²ÄĮĻ£¬ÓŠÓÅŅģµÄÄĶÄ„ŠŌ”£ĖüĆĒµÄ¾§Ģå½į¹¹ČēÓŅĶ¼ĖłŹ¾”£
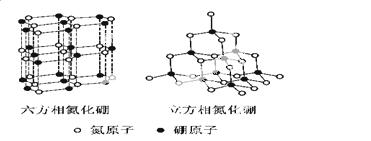
¢Å»łĢ¬ÅšŌ×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£
¢Ę ¹ŲÓŚÕāĮ½ÖÖ¾§ĢåµÄĖµ·Ø£¬ÕżČ·µÄŹĒ (ĢīŠņŗÅ)”£
a.Į¢·½ĻąµŖ»ÆÅšŗ¬ÓŠ¦Ņ¼üŗĶ¦Š¼ü£¬ĖłŅŌÓ²¶Č“ó b.Įł·½ĻąµŖ»ÆÅš²ć¼ä×÷ÓĆĮ¦Š”£¬ĖłŅŌÖŹµŲČķ
c.Į½ÖÖ¾§ĢåÖŠµÄB£N¼ü¾łĪŖ¹²¼Ū¼ü d.Į½ÖÖ¾§Ģå¾łĪŖ·Ö×Ó¾§Ģå
¢ĒĮł·½ĻąµŖ»ÆÅš¾§Ģå²ćÄŚŅ»øöÅšŌ×ÓÓėĻąĮŚµŖŌ×Ó¹¹³ÉµÄæռ乹ŠĶĪŖ £¬Ęä½į¹¹ÓėŹÆÄ«ĻąĖĘČ“²»µ¼µē£¬ŌŅņŹĒ ”£
¢ČĮ¢·½ĻąµŖ»ÆÅš¾§ĢåÖŠ£¬ÅšŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ ”£øĆ¾§ĢåµÄĢģČ»æóĪļŌŚĒą²ŲøßŌŌŚĻĀŌ¼300KmµÄ¹ÅµŲæĒÖŠ±»·¢ĻÖ”£øł¾ŻÕāŅ»æóĪļŠĪ³ÉŹĀŹµ£¬ĶʶĻŹµŃéŹŅÓÉĮł·½ĻąµŖ»ÆÅšŗĻ³ÉĮ¢·½ĻąµŖ»ÆÅšŠčŅŖµÄĢõ¼žÓ¦ŹĒ ”£
¢ÉNH4BF4(·śÅšĖįļ§)ŹĒŗĻ³ÉµŖ»ÆÅšÄÉĆ׹ܵÄŌĮĻÖ®Ņ»”£1mo NH4BF4ŗ¬ÓŠ molÅäĪ»¼ü”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬±ķÖŠĖłĮŠµÄ×ÖÄø·Ö±š“ś±ķŅ»ÖÖ»ÆѧŌŖĖŲ”£
(1)Š“³öÓÉÉĻŹöĮ½ÖÖŌŖĖŲ×é³ÉX2Y2ŠĶ»ÆŗĻĪļµÄ»ÆѧŹ½·Ö±šĪŖ____________£¬Ę侧ĢåĄąŠĶ·Ö±šĪŖ__________________”£
(2)B”¢C”¢DµÄĒā»ÆĪļÖŠ£¬·Šµć×īøßµÄĒā»ÆĪļµÄ½į¹¹Ź½ŹĒ____________£¬ø»ŗ¬·Šµć×īµĶµÄĒā»ÆĪļµÄæóĪļµÄĆū³ĘŹĒ____________”£
(3)ÉĻŹöŌŖĖŲŠĪ³ÉµÄµ„ÖŹÖŠ£¬ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ________(ĢīĆū³Ę)£¬Ęäæռ乹ŠĶĪŖ________________”£
(4)Š“³öÓÉÉĻŹöŌŖĖŲÖŠĻąĶ¬µÄĖÄÖÖŌŖĖŲ×é³ÉµÄĮ½ÖÖ»ÆŗĻĪļµÄĖ®ČÜŅŗ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____________________”£
(5)Š“³öÓÉÉĻŹöŌŖĖŲÖŠµÄČżÖÖŠĪ³ÉµÄ¼Čŗ¬ÓŠĄė×Ó¼üÓÖŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üµÄ»ÆŗĻĪļµÄµē×ÓŹ½£ŗ____________________(ČĪŠ“Į½øö)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ō×ÓŠņŹżŅĄ“ĪŌö“óµÄĖÄÖÖŌŖĖŲA”¢B”¢C”¢D·Ö±š“¦ÓŚµŚŅ»ÖĮµŚĖÄÖÜĘŚ£¬×ŌČ»½ēÖŠ“ęŌŚ¶ąÖÖAµÄ»ÆŗĻĪļ£¬BŌ×ÓŗĖĶāµē×ÓÓŠ6ÖÖ²»Ķ¬µÄŌĖ¶ÆדĢ¬£¬BÓėCæÉŠĪ³ÉÕżĖÄĆęĢåŠĪ·Ö×Ó£¬DµÄ»łĢ¬Ō×ÓµÄ×īĶāÄܲćÖ»ÓŠŅ»øöµē×Ó£¬ĘäĖūÄÜ²ć¾łŅŃ³äĀśµē×Ó”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÕāĖÄÖÖŌŖĖŲÖŠµēøŗŠŌ×ī“óµÄŌŖĖŲ£¬Ę仳Ģ¬Ō×ӵļŪµē×ÓÅŲ¼Ķ¼ĪŖ________£¬µŚŅ»µēĄėÄÜ×īŠ”µÄŌŖĖŲŹĒ________(ĢīŌŖĖŲ·ūŗÅ)”£
(2)CĖłŌŚÖ÷×åµÄĒ°ĖÄÖÖŌŖĖŲ·Ö±šÓėAŠĪ³ÉµÄ»ÆŗĻĪļ£¬·ŠµćÓÉøßµ½µĶµÄĖ³ŠņŹĒ________(Ģī»ÆѧŹ½)£¬³ŹĻÖČē“ĖµŻ±ä¹ęĀɵÄŌŅņŹĒ___________________”£
(3)BŌŖĖŲæÉŠĪ³É¶ąÖÖµ„ÖŹ£¬Ņ»ÖÖ¾§Ģå½į¹¹ČēĶ¼Ņ»ĖłŹ¾£¬ĘäŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ________”£ĮķŅ»Öֵľ§°ūČēĶ¼¶žĖłŹ¾£¬Čō“Ė¾§°ūÖŠµÄĄā³¤ĪŖ356.6 pm£¬Ōņ“Ė¾§°ūµÄĆܶČĪŖ________________________________________________g·c m£3(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£(
m£3(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£(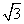 £½1.732)
£½1.732)
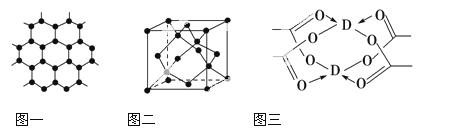
(4)DŌŖĖŲŠĪ³ÉµÄµ„ÖŹ£¬Ę侧ĢåµÄ¶Ń»żÄ£ŠĶĪŖ________£¬DµÄ“×ĖįŃĪ¾§Ģå¾Ö²æ½į¹¹ČēĶ¼Čż£¬øĆ¾§ĢåÖŠŗ¬ÓŠµÄ»Æѧ¼üŹĒ________(ĢīŃ”ĻīŠņŗÅ)”£
¢Ł¼«ŠŌ¼ü””””¢Ś·Ē¼«ŠŌ¼ü””””¢ŪÅäĪ»¼ü””””¢Ü½šŹō¼ü
(5)ĻņDµÄĮņĖįŃĪČÜŅŗÖŠµĪ¼Ó¹żĮæ°±Ė®£¬¹Ū²ģµ½µÄĻÖĻóŹĒ________”£ĒėŠ“³öÉĻŹö¹ż³ĢµÄĄė×Ó·½³ĢŹ½£ŗ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČżøöĆܱÕČŻĘ÷ÖŠ·Ö±š³äČėNe”¢H2”¢O2ČżÖÖĘųĢ壬µ±ĖüĆĒµÄĪĀ¶ČŗĶĆܶȶ¼ĻąĶ¬Ź±£¬ÕāČżÖÖĘųĢåµÄŃ¹Ēæ“ӓ󵽊”µÄĖ³ŠņŹĒ
A£®p(Ne) £¾p(H2) £¾p(O2) B£®p(O2) £¾p(Ne) £¾p(H2)
C£®p(H2) £¾p(O2) £¾p(Ne) D£®p(H2) £¾p(Ne) £¾p(O2)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®lmol FeI2Óė×ćĮæĀČĘų·“Ó¦Ź±×ŖŅʵĵē×ÓŹżĪŖ2NA
B£®2 L0.5 mol • L£1ĮņĖį¼ŲČÜŅŗÖŠŅõĄė×ÓĖł“ųµēŗÉŹżĪŖNA
C£®1 mol Na2O2¹ĢĢåÖŠŗ¬Ąė×Ó×ÜŹżĪŖ4NA
D£®±ūĻ©ŗĶ»·±ūĶé×é³ÉµÄ42 g»ģŗĻĘųĢåÖŠĒāŌ×ÓµÄøöŹżĪŖ6  NA
NA
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com