
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��.���������ϡ�
(1)Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡ���ᡣ
��.���Ʊ���Ʒ��
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
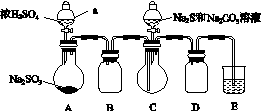
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�Ļ����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�еĻ�����Һ��________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.��̽���뷴˼��
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡ���ᡢ����ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________________________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����______________________________________________________________________________��
(3)Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��________�����ᴿ��
��.(1)�ٷ�Һ©������B
(3)����
��.(1)���ˣ�������ˮϴ�ӳ�����������м�������ϡ���ᡡ(2)����A����ƿ�μ�ŨH2SO4�����������彫װ���еĿ����ž�������C����ƿ����Na2S��Na2CO3�����Һ
(3)�ؽᾧ
[����] ��.(1)��aΪ��Һ©������E���Լ���������SO2β������ΪNaOH��Һ��(3)����Na2S2O3��5H2O����ɫ�����壬������ˮ����Ҫ����Һ�еõ���������ƾ��壬�辭������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�����õ���Ʒ����. (1)Na2S2O3��5H2O��ϡ��Һ��BaCl2����������ɣ���ʵ��������а�ɫ�������ɣ������һ����֤�������ɫ�����еμ�ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��(2)Na2SO3�����ױ������е�����������Na2SO4��Ϊ�˼���Na2SO4���ɵ��������ſ�װ���еĿ�����(3)Na2S2O3��5H2O���ܽ�����¶������������ᾧʱ���������������ʣ����ͨ���ؽᾧ�ķ����ᴿ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ��������ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����(����)
A�������̬�⻯������ȶ��ԣ�R>Q
B������������Ӧˮ��������ԣ�Q<W
C��ԭ�Ӱ뾶��T>Q>R
D����T������Һһ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӻ��ȡ������ܡ����ܵĵ�λ����kJ��mol-1
| ���� | ����ԭ�ӻ��� | ���ӻ����� | ������ | ���ۼ� | ���� |
| Na | 108.4 | NaCl | 786 | Cl-Cl | 243 |
| Mg | 146.4 | NaBr | 747 | Si-Si | 176 |
| Al | 326.4 | MgO | 3791 | Si-Cl | 360 |
������˵����ȷ����
A��Na(s)��Cl2��g����Ӧ����1molNaCl(s)�ų�������Ϊ556.1kJ
B��Si(s)+2Cl2(g)=SiCl4(g) ��H= �� 602kJ��mol-1
C���ӱ��п��Կ������Ȼ��Ƶ��۵�Ⱦ�����
D���ӱ������ݿ��Կ��������뾶Խ������������Ӽ���Խ���������ۼ�ȴԽǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������γ���Ҫ������
A��ɭ����ҿ��ķ����ƻ�����̬���� B������ʯȼ�ϵĴ���ȼ��
C�������ж�����̼�ĺ������� D�������ŷŴ���β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ���ձ��ж�ʢ��ϡ���ᡣ
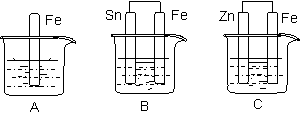 |
��1��A�з�Ӧ�����ӷ���ʽΪ ��
��2��B�еĵ缫��Ӧ��Fe�� ��Sn�� ��Sn��������Һ��pH��������С�䣩 ��
��3��C�б���ʴ�Ľ����� ����缫��ӦʽΪ ��
�Ƚ�A��B��C�д�������ʴ�������ɿ쵽����˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A����ƿ���������ȵķ�Ӧ��
B�������£����ܽ�Ũ����ʢ������Ͱ��
C��������Һ����ʱ����������ƿ�̶Ȼ�ʹ��ҺŨ��ƫ��
D��������ˮ��ʪ����ֽ����Һ��pH��һ����ʹ���ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
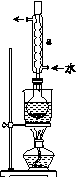
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s) Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O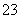 ��I2===S4O
��I2===S4O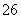 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
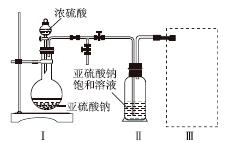
(1)װ�â��в�������Ļ�ѧ����ʽΪ______________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����______��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ________(�����)��
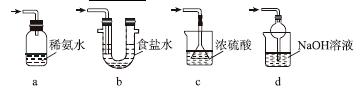
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH����b������Ba(OH)2��Һ
c���������� d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)���Na2S2O5�����ڿ������ѱ�������ʵ�鷽����
________________________________________________________________________
________________________________________________________________________��
ʵ���������Ѿ��п��������������IJⶨ
(6)���ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O===H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ________g��L��1��
��������ʵ������У����в���HI��������������ⶨ���________(�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯���������仯���� ( )
A�������ڷŵ������±�ɳ�����
B�������ڼ��������±�ɰ�ɫ��ˮ����ͭ
C��Ư�IJ�ñ���ÿ����б��
D���������������������þ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com